GPR15LG, a chemokine-like ligand for the G-protein coupled receptor 15 (GPR15), is abundantly expressed in the gastrointestinal mucosa and inflamed skin. Emerging evidence suggests its involvement in inflammatory disorders and cancers. C-X-C chemokine receptor type 4 (CXCR4) plays a critical role in immune cell trafficking and cancer metastasis. Recent evidence suggests a connection between GPR15LG and CXCR4 signaling, which has not been investigated so far.
We investigated the effects of GPR15LG on CXCR4 signaling and downstream functions. Binding assays and computational modeling were performed to assess the interaction between GPR15LG and CXCR4. Functional assays, including wound healing and cell migration assays, were conducted across various cell types, including CD4⁺ T cells and cancer cells, to evaluate the impact of GPR15LG on CXCL12-mediated CXCR4 signaling.
The results demonstrate that GPR15LG binds to the orthosteric site of CXCR4, modulating downstream signaling in a context-dependent manner. Specifically, GPR15LG enhances CXCL12-mediated CXCR4 signaling synergistically, promoting wound healing and cell migration across various cell types, including CD4 + T cells and cancer cells.
These findings underscore the role of GPR15LG in inflammation and metastasis, offering potential therapeutic avenues for CXCR4-mediated diseases.
© 2025. The Author(s).
Product Citations: 93
GPR15LG binds CXCR4 and synergistically modulates CXCL12-induced cell signaling and migration.
In Cell Communication and Signaling : CCS on 20 May 2025 by Albers, D. P. J., Novikova, S., et al.
-
Endocrinology and Physiology
In Scientific Reports on 28 March 2025 by Puig-Gamez, M., Van Attekum, M., et al.
Natural killer (NK) cells are prototypic cytotoxic innate lymphocytes that can kill target cells, such as tumor cells, in the absence of antigen-restriction. Peripheral NK cells exhibit a high degree of heterogeneity. Here, we set out to broadly assess intrinsic modulators of NK cell degranulation in an unbiased manner. We stimulated human primary blood-borne NK cells pre-treated with different cytokine regimens with the HCT116 human colon cancer cell line and used detection of lysosome-associated membrane glycoprotein 1 (LAMP1) as an identifier of rapid NK cell degranulation. RNA sequencing of FACS-sorted LAMP1hi NK cells showed CXCR4 and S1PR5 were top down-regulated genes. Using compounds that modulate activity of CXCR4 and S1P receptor family members S1P1 and S1P5, we confirmed they play an important immunosuppressive role in NK cell cytotoxicity. Mechanistically, engagement of CXCR4 and S1P1/5 receptors triggered phosphorylation of p42 and Ca2+ influx. CXCR4 activation promoted S1P5 upregulation and vice versa, and joint activation of both receptors amplified the defect NK cell degranulation. Intriguingly, in tumor samples the expression of both receptors and the synthesis of their ligands themselves appear to be coordinately regulated. Together, these data suggest that specifically and simultaneously targeting CXCR4 and S1P5 activity in the tumor microenvironment (TME) could be a beneficial strategy to unleash full cytotoxic potential of cytotoxic NK effector cells in the tumor.
© 2025. The Author(s).
-
FC/FACS
-
Biochemistry and Molecular biology
-
Cancer Research
Enhanced or reversible RNA N6-methyladenosine editing by red/far-red light induction.
In Nucleic Acids Research on 27 February 2025 by Tang, H., Han, S., et al.
The RNA N6-methyladenosine (m6A) modification is a critical regulator of various biological processes, but precise and dynamic control of m6A remains a challenge. In this work, we present a red/far-red light-inducible m6A editing system that enables efficient and reversible modulation of m6A levels with minimal off-target effects. By engineering the CRISPR dCas13 protein and sgRNA with two pairs of light-inducible heterodimerizing proteins, ΔphyA/FHY1 and Bphp1/PspR2, we achieved targeted recruitment of m6A effectors. This system significantly enhances m6A writing efficiency and allows dynamic regulation of m6A deposition and removal on specific transcripts, such as SOX2 and ACTB. Notably, reversible m6A editing was achieved through cyclic modulation at a single target site, demonstrating the ability to influence mRNA expression and modulate the differentiation state of human embryonic stem cells. This optogenetic platform offers a precise, versatile tool for cyclic and reversible m6A regulation, with broad implications for understanding RNA biology and its potential applications in research and medicine.
© The Author(s) 2025. Published by Oxford University Press on behalf of Nucleic Acids Research.
-
Biochemistry and Molecular biology
-
Genetics
In Cancers on 27 January 2025 by Kwiecień, I., Rutkowska, E., et al.
The presence of metastases in mediastinal lymph nodes (LNs) is essential for planning lung cancer treatment and assessing anticancer immune responses. The aim of the study was to assess LNs for the presence of neoplastic cells and evaluate lung cancer-selected antigen expression. LN aspirates were obtained during an EBUS/TBNA procedure. The cells were analyzed using a hematological analyzer and flow cytometry. It was possible to indicate the presence of cells characterized by high fluorescence connected with high metabolic activity using a hematological analyzer and to determine their non-hematopoietic origin using flow cytometry. Using these methods together, we detected very quickly a high proportion of cancer cells in LNs. We noticed that it was possible to determine a high expression of EpCAM, TTF-1, Ki67, cytokeratin, HER, and differences between non-small-cell (NSCLC) and small-cell lung cancer (SCLC) for the antigens MUC-1, CD56, HLA-DR, CD39, CD184, PD-L1, PD-L2 and CTLA-4 on tumor cells. We report, for the first time, that the detection of tumor cells in LNs with the expression of specific antigens is easy to evaluate using a hematological analyzer and flow cytometry in EBUS/TBNA samples. Such precise characteristics of non-hematopoietic cells in LNs may be of great diagnostic importance in the detection of micrometastases.
-
Cancer Research
In Stem Cell Research & Therapy on 2 September 2024 by Wang, S., Yu, Y., et al.
Understanding the lineage differentiation of human prostate not only is crucial for basic research on human developmental biology but also significantly contributes to the management of prostate-related disorders. Current knowledge mainly relies on studies on rodent models, lacking human-derived alternatives despite clinical samples may provide a snapshot at certain stage. Human embryonic stem cells can generate all the embryonic lineages including the prostate, and indeed a few studies demonstrate such possibility based on co-culture or co-transplantation with urogenital mesenchyme into mouse renal capsule.
To establish a stepwise protocol to obtain prostatic organoids in vitro from human embryonic stem cells, we apply chemicals and growth factors by mimicking the regulation network of transcription factors and signal transduction pathways, and construct cell lines carrying an inducible NKX3-1 expressing cassette, together with three-dimensional culture system. Unpaired t test was applied for statistical analyses.
We first successfully generate the definitive endoderm, hindgut, and urogenital sinus cells. The embryonic stem cell-derived urogenital sinus cells express prostatic key transcription factors AR and FOXA1, but fail to express NKX3-1. Therefore, we construct NKX3-1-inducible cell line by homologous recombination, which is eventually able to yield AR, FOXA1, and NKX3-1 triple-positive urogenital prostatic lineage cells through stepwise differentiation. Finally, combined with 3D culture we successfully derive prostate-like organoids with certain structures and prostatic cell populations.
This study reveals the crucial role of NKX3-1 in prostatic differentiation and offers the inducible NKX3-1 cell line, as well as provides a stepwise differentiation protocol to generate human prostate-like organoids, which should facilitate the studies on prostate development and disease pathogenesis.
© 2024. The Author(s).
-
Homo sapiens (Human)
-
Stem Cells and Developmental Biology
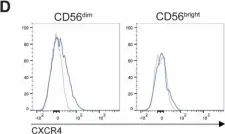
In Sci Rep on 28 March 2025 by Puig-Gamez, M., Van Attekum, M., et al.
Fig.3.D

-
FC/FACS
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 4
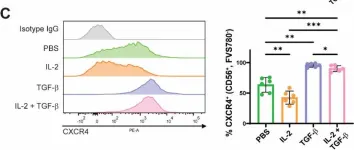
In Sci Rep on 28 March 2025 by Puig-Gamez, M., Van Attekum, M., et al.
Fig.3.C

-
FC/FACS
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 4
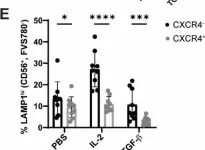
In Sci Rep on 28 March 2025 by Puig-Gamez, M., Van Attekum, M., et al.
Fig.3.E

-
FC/FACS
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 4
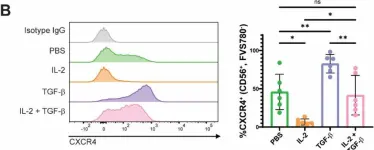
In Sci Rep on 28 March 2025 by Puig-Gamez, M., Van Attekum, M., et al.
Fig.3.B

-
FC/FACS
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 4