Glioblastoma (GB) is a grade IV glial tumor characterized by high malignancy and dismal prognosis, primarily due to high recurrence rates and therapeutic resistance. The epidermal growth factor receptor (EGFR), a receptor tyrosine kinase (RTK), regulates signaling pathways, including cell growth, proliferation, survival, migration, and cell death. Many cancers utilize immune checkpoints (ICs) to attenuate immune responses. CD73 is an enzyme that functions as an IC by hydrolyzing AMP to adenosine, suppressing immune cells in the tumor microenvironment. However, the role of CD73 in resistance to EGFR inhibitors is poorly understood. This study aims to elucidate the resistance mechanisms induced by anti-EGFR treatment and to evaluate an anti-CD73 approach to overcome resistance mediated by anti-EGFR monotherapy.
The U251 GB cell line was treated with AG1478, an EGFR inhibitor, and the resistance markers MRP-1, PD-L1, and CD73 were evaluated using flow cytometry. Additionally, we assessed the combination effects of AG1478 and APCP (an EGFR and a CD73 inhibitor, respectively) on cell cycle progression, proliferation, apoptosis, and migration in vitro.
We observed high EGFR, PD-L1, and CD73 expression in human GB cells. The treatment with AG1478 increased the expression of resistance markers MRP-1, PD-L1, and CD73, whereas it decreased CTLA-4. The combination of AG1478 and APCP did not alter proliferation or apoptosis but interfered with cell cycling, arresting the cells in the G1 phase, decreasing cell motility and partially reversing MRP-1 overexpression.
In summary, our findings indicate that CD73 inhibition has a modest effect in overcoming resistance to EGFR monotherapy in vitro. Thus, further in vivo studies are needed, as the inhibition of both EGFR and CD73 affects cells in the tumor microenvironment and could potentially enhance anti-tumor immunity.
© 2025 The Authors.
Product Citations: 29
Assessing the impact of CD73 inhibition on overcoming anti-EGFR resistance in glioma cells.
In Oncology Research on 7 April 2025 by Silva, L. F. L., Scholl, J. N., et al.
-
Cancer Research
In Cells on 11 February 2025 by Courot, H., Rigal, E., et al.
Glioblastomas (GBMs) are lethal brain tumors in which EGFR gene amplification or mutation is frequently detected and is associated with poor prognosis. The standard of care is maximal resection followed by chemotherapy and radiation. Over the last twenty years, marginal improvements in patient survival have been achieved mainly through surgical techniques and the more accurate use of radiation. In this study, umbilical cord blood-derived and expanded human allogeneic natural killer (eNK) cells were pre-complexed to an Fc-engineered anti-EGFR monoclonal antibody (Pin-EGFR) to create Pin-EGFR-armed eNK cells. Pin-EGFR-armed eNK cells showed in vitro persistence of mAb anchoring. This arming process mediated specific, rapid and potent NK cell-redirected cytotoxicity against GBM cell lines and patient-derived cells in models consistent with the pathophysiological conditions of GBM. These results demonstrate the potential of Pin-EGFR-armed eNK cells to be an effective therapy against GBM cell lines in vitro. This product represents a promising strategy to directly target residual tumor tissue remaining at and beyond the resection margins immediately following GBM surgery to improve patient care.
-
Cell Biology
In Cell Rep Methods on 22 May 2023 by Mattsson, J., Ljungars, A., et al.
Phenotypic drug discovery (PDD) enables the target-agnostic generation of therapeutic drugs with novel mechanisms of action. However, realizing its full potential for biologics discovery requires new technologies to produce antibodies to all, a priori unknown, disease-associated biomolecules. We present a methodology that helps achieve this by integrating computational modeling, differential antibody display selection, and massive parallel sequencing. The method uses the law of mass action-based computational modeling to optimize antibody display selection and, by matching computationally modeled and experimentally selected sequence enrichment profiles, predict which antibody sequences encode specificity for disease-associated biomolecules. Applied to a phage display antibody library and cell-based antibody selection, ∼105 antibody sequences encoding specificity for tumor cell surface receptors expressed at 103-106 receptors/cell were discovered. We anticipate that this approach will be broadly applicable to molecular libraries coupling genotype to phenotype and to the screening of complex antigen populations for identification of antibodies to unknown disease-associated targets.
© 2023 The Author(s).
In Pharmaceuticals (Basel, Switzerland) on 21 March 2022 by Barrios-Bernal, P., Hernández-Pedro, N., et al.
The combination of metformin and TKIs for non-small cell lung cancer has been proposed as a strategy to overcome resistance of neoplastic cells induced by several molecular mechanisms. This study sought to investigate the effects of a second generation TKI afatinib, metformin, or their combination on three adenocarcinoma lung cancer cell lines with different EGFRmutation status. A549, H1975, and HCC827 cell lines were treated with afatinib, metformin, and their combination for 72 h. Afterwards, several parameters were assessed including cytotoxicity, interactions, apoptosis, and EGFR protein levels at the cell membrane and several glycolytic, oxidative phosphorylation (OXPHOS), and EMT expression markers. All cell lines showed additive to synergic interactions for the induction of cytotoxicity caused by the tested combination, as well as an improved pro-apoptotic effect. This effect was accompanied by downregulation of glycolytic, EMT markers, a significant decrease in glucose uptake, extracellular lactate, and a tendency towards increased OXPHOS subunits expression. Interestingly, we observed a better response to the combined therapy in lung cancer cell lines A549 and H1975, which normally have low affinity for TKI treatment. Findings from this study suggest a sensitization to afatinib therapy by metformin in TKI-resistant lung cancer cells, as well as a reduction in cellular glycolytic phenotype.
-
FC/FACS
-
Homo sapiens (Human)
-
Cancer Research
Glioblastoma mutations alter EGFR dimer structure to prevent ligand bias.
In Nature on 1 February 2022 by Hu, C., Leche, C. A., et al.
The epidermal growth factor receptor (EGFR) is frequently mutated in human cancer1,2, and is an important therapeutic target. EGFR inhibitors have been successful in lung cancer, where mutations in the intracellular tyrosine kinase domain activate the receptor1, but not in glioblastoma multiforme (GBM)3, where mutations occur exclusively in the extracellular region. Here we show that common extracellular GBM mutations prevent EGFR from discriminating between its activating ligands4. Different growth factor ligands stabilize distinct EGFR dimer structures5 that signal with different kinetics to specify or bias outcome5,6. EGF itself induces strong symmetric dimers that signal transiently to promote proliferation. Epiregulin (EREG) induces much weaker asymmetric dimers that drive sustained signalling and differentiation5. GBM mutations reduce the ability of EGFR to distinguish EREG from EGF in cellular assays, and allow EGFR to form strong (EGF-like) dimers in response to EREG and other low-affinity ligands. Using X-ray crystallography, we further show that the R84K GBM mutation symmetrizes EREG-driven extracellular dimers so that they resemble dimers normally seen with EGF. By contrast, a second GBM mutation, A265V, remodels key dimerization contacts to strengthen asymmetric EREG-driven dimers. Our results argue for an important role of altered ligand discrimination by EGFR in GBM, with potential implications for therapeutic targeting.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
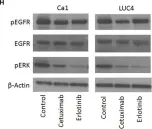
In Mol Oncol on 1 April 2021 by Qi, Z., Qiu, Y., et al.
Fig.1.C

-
WB
-
Collected and cropped from Mol Oncol by CiteAb, provided under a CC-BY license
Image 1 of 2
In Oncotarget on 2 March 2018 by Setúbal Destro Rodrigues, M. F., Gammon, L., et al.
Fig.2.H

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 2