The placenta is an essential organ of pregnancy required for maternal-fetal transport and communication. The surface of the placenta facing the maternal blood is formed by a single giant multinucleate cell: the syncytiotrophoblast. The syncytiotrophoblast is formed and maintained via fusion of progenitor cytotrophoblasts. Cell-cell fusion is a tightly regulated process, and in non-trophoblastic cells is accompanied by stereotypical alterations in cell shape by cells that have attained fusion-competence. The most prominent feature is the formation of actin-based membrane protrusions, but whether stereotypic morphological changes occur in fusion-competent cytotrophoblasts has not been characterized. Using a human placental explant model and trophoblast organoids, we identify microvilliation as a morphological feature that is enriched prior to fusion of cytotrophoblasts. Disruption of microvilli using an inhibitor of the actin-membrane cross-linker protein ezrin blocked cytotrophoblast fusion in both models. We provide evidence that cytotrophoblast microvilli are enriched in early endosomes and a pro-fusogenic protein. Thus, we propose that the polarized assembly of microvillar domains is crucial for mediating efficient syncytiotrophoblast development.
© 2025. Published by The Company of Biologists.
Product Citations: 13
Placental cytotrophoblast microvillar stabilization is required for cell-cell fusion.
In Development (Cambridge, England) on 1 April 2025 by Duan, W., Shaha, S., et al.
-
Stem Cells and Developmental Biology
TXNIP mediates LAT1/SLC7A5 endocytosis to reduce amino acid uptake in cells entering quiescence
Preprint on BioRxiv : the Preprint Server for Biology on 30 October 2024 by Kahlhofer, J., Marchet, N., et al.
Entry and exit from cellular quiescence require dynamic adjustments in nutrient acquisition, yet the mechanisms by which quiescent cells downregulate amino acid (AA) transport remain poorly understood. Here, we demonstrate that cells entering quiescence select plasma membrane-resident AA transporters for endocytosis and lysosomal degradation, to match AA uptake with reduced translation. We identify the α-arrestin TXNIP as a key regulator of AA uptake during quiescence, since it mediates the endocytosis of the SLC7A5-SLC3A2 (LAT1-4F2hc) transporter complex in response to reduced AKT signaling. Mechanistically, TXNIP interacts with HECT-type ubiquitin ligases to facilitate transporter ubiquitination. Loss of TXNIP disrupts this regulation, resulting in dysregulated AA uptake, sustained mTORC1 signaling, and accelerated quiescence exit. A novel TXNIP loss-of-function mutation in a patient with severe metabolic disease further supports its role in nutrient homeostasis and human health. These findings highlight TXNIP’s role in controlling SLC7A5-SLC3A2 mediated AA acquisition with implications for quiescence biology and disease.
In Cell Reports Medicine on 21 May 2024 by Glass, D. R., Mayer-Blackwell, K., et al.
Cutaneous T cell lymphomas (CTCLs) are skin cancers with poor survival rates and limited treatments. While immunotherapies have shown some efficacy, the immunological consequences of administering immune-activating agents to CTCL patients have not been systematically characterized. We apply a suite of high-dimensional technologies to investigate the local, cellular, and systemic responses in CTCL patients receiving either mono- or combination anti-PD-1 plus interferon-gamma (IFN-γ) therapy. Neoplastic T cells display no evidence of activation after immunotherapy. IFN-γ induces muted endogenous immunological responses, while anti-PD-1 elicits broader changes, including increased abundance of CLA+CD39+ T cells. We develop an unbiased multi-omic profiling approach enabling discovery of immune modules stratifying patients. We identify an enrichment of activated regulatory CLA+CD39+ T cells in non-responders and activated cytotoxic CLA+CD39+ T cells in leukemic patients. Our results provide insights into the effects of immunotherapy in CTCL patients and a generalizable framework for multi-omic analysis of clinical trials.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
-
Homo sapiens (Human)
-
Cancer Research
-
Immunology and Microbiology
Plasmodium vivax binds host CD98hc (SLC3A2) to enter immature red blood cells.
In Nature Microbiology on 1 August 2021 by Malleret, B., El Sahili, A., et al.
More than one-third of the world's population is exposed to Plasmodium vivax malaria, mainly in Asia1. P. vivax preferentially invades reticulocytes (immature red blood cells)2-4. Previous work has identified 11 parasite proteins involved in reticulocyte invasion, including erythrocyte binding protein 2 (ref. 5) and the reticulocyte-binding proteins (PvRBPs)6-10. PvRBP2b binds to the transferrin receptor CD71 (ref. 11), which is selectively expressed on immature reticulocytes12. Here, we identified CD98 heavy chain (CD98), a heteromeric amino acid transporter from the SLC3 family (also known as SLCA2), as a reticulocyte-specific receptor for the PvRBP2a parasite ligand using mass spectrometry, flow cytometry, biochemical and parasite invasion assays. We characterized the expression level of CD98 at the surface of immature reticulocytes (CD71+) and identified an interaction between CD98 and PvRBP2a expressed at the merozoite surface. Our results identify CD98 as an additional host membrane protein, besides CD71, that is directly associated with P. vivax reticulocyte tropism. These findings highlight the potential of using PvRBP2a as a vaccine target against P. vivax malaria.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
-
Cardiovascular biology
In Cell Death & Disease on 7 November 2019 by Young, M. M., Bui, V., et al.
FTY720 (fingolimod) is a FDA-approved sphingosine analog that is phosphorylated in vivo to modulate sphingosine-1-phosphate receptor (S1PR) signaling for immunosuppression in patients with refractory multiple sclerosis. FTY720 also exhibits promising anticancer efficacy in several preclinical models. While FTY720-induced cytotoxicity is not due to S1PR signaling, the mechanism remains unclear and is reported to occur through various cell death pathways. Here, we performed a systematic, mechanistic study of FTY720-induced cell death in acute myeloid leukemia (AML). We found that FTY720 induced cell death in a panel of genetically diverse AML cell lines that was accompanied by rapid phosphatidylserine (PS) externalization. Importantly, FTY720-induced PS exposure was not due to any direct effects on plasma membrane integrity and was independent of canonical signaling by regulated cell death pathways known to activate lipid flip-flop, including caspase-dependent apoptosis/pyroptosis, necroptosis, ferroptosis, and reactive oxygen species-mediated cell death. Notably, PS exposure required cellular vacuolization induced by defects in endocytic trafficking and was suppressed by the inhibition of PP2A and shedding of Annexin V-positive subcellular particles. Collectively, our studies reveal a non-canonical pathway underlying PS externalization and cell death in AML to provide mechanistic insight into the antitumor properties of FTY720.
-
Homo sapiens (Human)
-
Cancer Research
-
Cell Biology
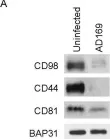
In PLoS One on 10 November 2017 by Viswanathan, K., Verweij, M. C., et al.
Fig.2.A

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 1