One of the puzzling aspects of sporadic Alzheimer's disease (AD) is how it commences. Changes in one key brain peptide, amyloid-beta (Aβ), accompany disease progression, but whether this comprises a trigger or a consequence of AD is still a topic of debate. It is clear however that the cerebral presence of oligomeric Aβ (1-42) is a key factor in early AD-pathogenesis. Furthermore, treatment of rodent brains with oligomeric Aβ (1-42) either in vitro or in vivo, acutely impairs hippocampal synaptic plasticity, creating a link between Aβ-pathology and learning impairments. Here, we show that a once-off inoculation of the brains of healthy adult rats with oligomeric Aβ (1-42) exerts debilitating effects on the long-term viability of the hippocampus, one of the primary targets of AD. Changes are progressive: months after treatment, synaptic plasticity, neuronal firing and spatial learning are impaired and expression of plasticity-related proteins are changed, in the absence of amyloid plaques. Early changes relate to activation of microglia, whereas later changes are associated with a reconstruction of astroglial morphology. These data suggest that a disruption of Aβ homeostasis may suffice to trigger an irreversible cascade, underlying progressive loss of hippocampal function, that parallels the early stages of AD.
Copyright © 2024 Kramer, Hoang, Yang, Shchyglo, Böge, Neubacher, Colitti-Klausnitzer and Manahan-Vaughan.
Product Citations: 155
In Frontiers in Aging Neuroscience on 19 August 2024 by Kramer, M., Hoang, T. H., et al.
A Novel Simple ImmunoAssay for Quantification of Blood Anti-NMDAR1 Autoantibodies
Preprint on BioRxiv : the Preprint Server for Biology on 15 April 2024 by Vaughn, M., Powell, S., et al.
High titers of anti-NMDAR1 autoantibodies in human brain cause anti-NMDAR1 encephalitis, a rare disease that displays a variety of psychiatric symptoms and neurological symptoms. Currently, immunohistochemical staining and cell-based assays are the standard methods for detection and semi-quantification of the anti-NMDAR1 autoantibodies. Low titers of blood circulating anti-NMDAR1 autoantibodies have been reported in a significant subset of the general human population. However, detection and quantification of these low titers of blood circulating anti-NMDAR1 autoantibodies are problematic because of high non-specific background from less diluted serum/plasma. Development of a new method to quantify these low titers of blood anti-NMDAR1 autoantibodies is necessary to understand their potential impacts on psychiatric symptoms and cognition. Based on our previous One-Step assay, we report the development of a novel simple immunoassay to quantify cross-species blood anti-NMDAR1 autoantibodies, and its validation with immunohistochemistry and cell-based assays in both humans and mice.
-
Cardiovascular biology
In PLoS ONE on 21 March 2024 by Bonanni, R., Cariati, I., et al.
Neuronal death could be responsible for the cognitive impairments found in astronauts exposed to spaceflight, highlighting the need to identify potential countermeasures to ensure neuronal health in microgravity conditions. Therefore, differentiated HT22 cells were exposed to simulated microgravity by random positioning machine (RPM) for 48 h, treating them with a single administration of Trolox, recombinant irisin (r-Irisin) or both. Particularly, we investigated cell viability by MTS assay, Trypan Blue staining and western blotting analysis for Akt and B-cell lymphoma 2 (Bcl-2), the intracellular increase of reactive oxygen species (ROS) by fluorescent probe and NADPH oxidase 4 (NOX4) expression, as well as the expression of brain-derived neurotrophic factor (BDNF), a major neurotrophin responsible for neurogenesis and synaptic plasticity. Although both Trolox and r-Irisin manifested a protective effect on neuronal health, the combined treatment produced the best results, with significant improvement in all parameters examined. In conclusion, further studies are needed to evaluate the potential of such combination treatment in counteracting weightlessness-induced neuronal death, as well as to identify other potential strategies to safeguard the health of astronauts exposed to spaceflight.
Copyright: © 2024 Bonanni et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Behavioral analysis of kainate receptor KO mice and the role of GluK3 subunit in anxiety.
In Scientific Reports on 24 February 2024 by Iida, I., Konno, K., et al.
Kainate receptors (KARs) are one of the ionotropic glutamate receptors in the central nervous system (CNS) comprised of five subunits, GluK1-GluK5. There is a growing interest in the association between KARs and psychiatric disorders, and there have been several studies investigating the behavioral phenotypes of KAR deficient mice, however, the difference in the genetic background has been found to affect phenotype in multiple mouse models of human diseases. Here, we examined GluK1-5 single KO mice in a pure C57BL/6N background and identified that GluK3 KO mice specifically express anxiolytic-like behavior with an alteration in dopamine D2 receptor (D2R)-induced anxiety, and reduced D2R expression in the striatum. Biochemical studies in the mouse cortex confirmed that GluK3 subunits do not assemble with GluK4 and GluK5 subunits, that can be activated by lower concentration of agonists. Overall, we found that GluK3-containing KARs function to express anxiety, which may represent promising anti-anxiety medication targets.
© 2024. The Author(s).
Preprint on BioRxiv : the Preprint Server for Biology on 8 January 2024 by Strauch, C., Böge, J., et al.
The entorhinal cortex sends afferent information to the hippocampus by means of the perforant path(PP), whereby the medial PP (MPP) is believed to convey information about spatial context and the lateral PP (LPP) may convey information about item identity. This information is encoded by means of synaptic plasticity. The PP input to the dentate gyrus(DG) terminates in the suprapyramidal (upper/inner) and infrapyramidal (lower/outer) blades. To what extent frequency-dependent synaptic plasticity in these blades differs is unclear. Here, we compared MPP-DG responses in the supra- (sDG) and infrapyramidal blades (iDG) of freely behaving adult rats and found that synaptic plasticity in the sDG is broadly frequency-dependent whereby long-term depression (LTD, >24h) is induced with stimulation at 1Hz, short-term depression (<2h) is triggered by 5 or 10Hz and long-term potentiation (LTP) of increasing magnitudes is induced by 200 and 400 Hz stimulation, respectively. By contrast, although the iDG expresses STD following 5 or 10Hz stimulation, LTD induced by 1Hz is weaker, LTP is not induced by 200Hz and LTP induced by 400Hz stimulation is significantly smaller in magnitude and is less persistent (<4h) compared to LTP in sDG. Furthermore, the stimulus-response relationship of the iDG is suppressed compared to sDG. Patch clamp recordings, in vitro, revealed reduced firing frequencies in response to high currents, and different action potential thresholds in iDG compared to sDG. Assessment of the expression of GluN subunits revealed significantly lower expression levels of GluN1, GluN2A and GluN2B in iDG compared to sDG. Taken together, these data indicate that synaptic plasticity in the infrapyramidal blade of the dentate gyrus is weaker, less persistent and less responsive to afferent frequencies than synaptic plasticity in sDG. Effects may be mediated by weaker NMDA receptor expression in iDG. These characteristics may explain reported differences in experience-dependent information processing in sDG versus iDG.
-
Rattus norvegicus (Rat)
-
Neuroscience
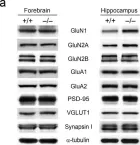
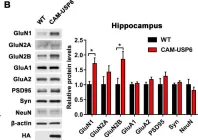
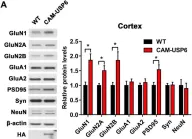
In Exp Mol Med on 1 August 2022 by Bae, Y. S., Yoon, S. H., et al.
Fig.1.A

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Exp Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 8
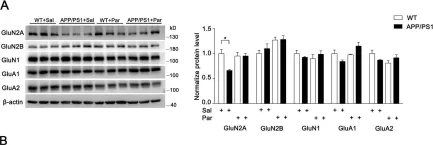
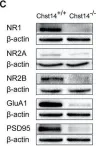
In Transl Neurodegener on 12 May 2020 by Ai, P. H., Chen, S., et al.
Fig.3.A

-
WB
-
Collected and cropped from Transl Neurodegener by CiteAb, provided under a CC-BY license
Image 1 of 8
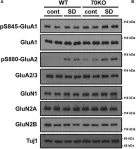
In Front Cell Neurosci on 24 January 2020 by Mayanagi, T. & Sobue, K.
Fig.4.A

-
WB
-
Collected and cropped from Front Cell Neurosci by CiteAb, provided under a CC-BY license
Image 1 of 8
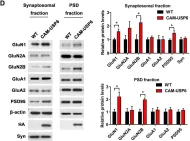
In PLoS Biol on 1 December 2019 by Zeng, F., Ma, X., et al.
Fig.5.D

-
WB
-
Collected and cropped from PLoS Biol by CiteAb, provided under a CC-BY license
Image 1 of 8
In PLoS Biol on 1 December 2019 by Zeng, F., Ma, X., et al.
Fig.5.B

-
WB
-
Collected and cropped from PLoS Biol by CiteAb, provided under a CC-BY license
Image 1 of 8
In PLoS Biol on 1 December 2019 by Zeng, F., Ma, X., et al.
Fig.5.A

-
WB
-
Collected and cropped from PLoS Biol by CiteAb, provided under a CC-BY license
Image 1 of 8
In Front Mol Neurosci on 12 March 2019 by Li, Q., Wu, X., et al.
Fig.3.C

-
WB
-
Collected and cropped from Front Mol Neurosci by CiteAb, provided under a CC-BY license
Image 1 of 8
In Eneuro on 9 December 2017 by Zhang, L., Jablonski, A. M., et al.
Fig.7.B

-
WB
-
Rattus norvegicus (Rat)
Collected and cropped from Eneuro by CiteAb, provided under a CC-BY license
Image 1 of 8