Almost all individuals with Down Syndrome (DS) develop Alzheimer's disease (AD) by mid to late life. However, the degree to which AD in DS shares pathological changes with sporadic late-onset AD (LOAD) and autosomal dominant AD (ADAD) beyond core AD biomarkers such as amyloid-β (Aβ) and tau is unknown. Here, we used proteomics of cerebrospinal fluid from individuals with DS (n = 229) in the Down Alzheimer Barcelona Neuroimaging Initiative (DABNI) cohort to assess the evolution of AD pathophysiology from asymptomatic to dementia stages and compared the proteomic biomarker changes in DS to those observed in LOAD and ADAD. Although many proteomic alterations were shared across DS, LOAD, and ADAD, DS demonstrated more severe changes in immune-related proteins, extracellular matrix pathways, and plasma proteins likely related to blood-brain barrier dysfunction compared to LOAD. These changes were present in young adults with DS prior to the onset of Aβ or tau pathology, suggesting they are associated with trisomy 21 and may serve as risk factors for DSAD. DSAD showed an earlier increase in markers of axonal and white matter pathology and earlier changes in markers potentially associated with cerebral amyloid angiopathy compared to ADAD. The unique features of DSAD may have important implications for treatment strategies in this population.
© 2025. The Author(s).
Product Citations: 36
In Nature Communications on 1 July 2025 by Montoliu-Gaya, L., Bian, S., et al.
-
Neuroscience
In Cell Death & Disease on 22 April 2025 by Krzystek, T. J., Rathnayake, R., et al.
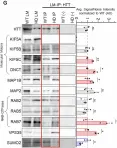
Huntington's disease (HD) is a devastating neurodegenerative disorder that manifests from an N-terminal polyQ-expansion (>35) in the Huntingtin (HTT) gene leading to axonal degeneration and significant neuronal death. Despite evidence for a scaffolding role for HTT in membrane-related processes such as endocytosis, vesicle transport, and vesicle fusion, it remains unclear how polyQ-expansion alters membrane binding during these processes. Using quantitative Mass Spectrometry-based proteomics on HTT-containing light vesicle membranes isolated from healthy and HD iPSC-derived neurons, we found significant changes in the proteome and kinome of signal transduction, neuronal translation, trafficking, and axon guidance-related processes. Through a combination of in vitro kinase assays, Drosophila genetics, and pharmacological inhibitors, we identified that GSK3β and ERK1 phosphorylate HTT and that these events play distinct and opposing roles during HD with inhibition of GSK3β decreasing polyQ-mediated axonal transport defects and neuronal cell death, while inhibition of ERK enhancing these phenotypes. Together, this work proposes two novel pathways in which GSK3β phosphorylation events exacerbate and ERK phosphorylation events mitigate HD-dependent neuronal dysfunction highlighting a highly druggable pathway for targeted therapeutics using already available small molecules.
© 2025. The Author(s).
-
WB
-
Cell Biology
In Autophagy on 1 January 2025 by Jia, N., Ganesan, D., et al.
Hyperphosphorylation and aggregation of MAPT (microtubule-associated protein tau) is a pathogenic hallmark of tauopathies and a defining feature of Alzheimer disease (AD). Pathological MAPT/tau is targeted by macroautophagy/autophagy for clearance after being sequestered within autophagosomes, but autophagy dysfunction is indicated in tauopathy. While mitochondrial bioenergetic deficits have been shown to precede MAPT/tau pathology in tauopathy brains, it is unclear whether energy metabolism deficiency is involved in the pathogenesis of autophagy defects. Here, we reveal that stimulation of anaplerotic metabolism restores defective oxidative phosphorylation (OXPHOS) in tauopathy neurons which, strikingly, leads to pronounced MAPT/tau clearance by boosting autophagy functionality through enhancements of mitochondrial biosynthesis and supply of phosphatidylethanolamine for autophagosome biogenesis. Furthermore, early anaplerotic stimulation of OXPHOS elevates autophagy activity and attenuates MAPT/tau pathology, thereby counteracting memory impairment in tauopathy mice. Taken together, our study sheds light on a pivotal role of mitochondrial bioenergetic deficiency in tauopathy-related autophagy defects and suggests a new therapeutic strategy to prevent the buildup of pathological MAPT/tau in AD and other tauopathy diseases.Abbreviation: AA: antimycin A; AD, Alzheimer disease; ATP, adenosine triphosphate; AV, autophagosome/autophagic vacuole; AZ, active zone; Baf-A1: bafilomycin A1; CHX, cycloheximide; COX, cytochrome c oxidase; DIV, days in vitro; DRG, dorsal root ganglion; ETN, ethanolamine; FRET, Förster/fluorescence resonance energy transfer; FTD, frontotemporal dementia; Gln, glutamine; HA: hydroxylamine; HsMAPT/Tau, human MAPT; IMM, inner mitochondrial membrane; LAMP1, lysosomal-associated membrane protein 1; LIs, lysosomal inhibitors; MDAV, mitochondria-derived autophagic vacuole; MmMAPT/Tau, murine MAPT; NFT, neurofibrillary tangle; OCR, oxygen consumption rate; Omy: oligomycin; OXPHOS, oxidative phosphorylation; PPARGC1A/PGC-1alpha: peroxisome proliferative activated receptor, gamma, coactivator 1 alpha; PE, phosphatidylethanolamine; phospho-MAPT/tau, hyperphosphorylated MAPT; PS, phosphatidylserine; PISD, phosphatidylserine decarboxylase;SQSTM1/p62, sequestosome 1; STX1, syntaxin 1; SYP, synaptophysin; Tg, transgenic; TCA, tricarboxylic acid; TEM, transmission electron microscopy.
-
Cell Biology
-
Neuroscience
In Molecular Neurobiology on 1 November 2024 by Cararo-Lopes, M. M., Sadovnik, R., et al.
α-Klotho (α-Kl) is a modulator of aging, neuroprotection, and cognition. Transcription of the Klotho gene produces two splice variants-a membrane protein (mKl), which can be cleaved and released into the extracellular milieu, and a truncated secreted form (sKl). Despite mounting evidence supporting a role for α-Kl in brain function, the specific roles of α-Kl isoforms in neuronal development remain elusive. Here, we examined α-Kl protein levels in rat brain and observed region-specific expression in the adult that differs between isoforms. In the developing hippocampus, levels of isoforms decrease after the third postnatal week, marking the end of the critical period for development. We overexpressed α-Kl isoforms in primary cultures of rat cortical neurons and evaluated effects on brain-derived neurotrophic factor (BDNF) signaling. Overexpression of either isoform attenuated BDNF-mediated signaling and reduced intracellular Ca2+ levels, with mKl promoting a greater effect. mKl or sKl overexpression in hippocampal neurons resulted in a partially overlapping reduction in secondary dendrite branching. Moreover, mKl overexpression increased primary dendrite number. BDNF treatment of neurons overexpressing sKl resulted in a dendrite branching phenotype similar to control neurons. In neurons overexpressing mKl, BDNF treatment restored branching of secondary and higher order dendrites close, but not distal, to the soma. Taken together, the data presented support the idea that sKl and mKl play distinct roles in neuronal development, and specifically, in dendrite morphogenesis.
© 2024. The Author(s).
-
Rattus norvegicus (Rat)
-
Immunology and Microbiology
In Nature Communications on 2 August 2024 by Galimberti, M., Nucera, M. R., et al.
Huntington's disease (HD) causes selective degeneration of striatal and cortical neurons, resulting in cell mosaicism of coexisting still functional and dysfunctional cells. The impact of non-cell autonomous mechanisms between these cellular states is poorly understood. Here we generated telencephalic organoids with healthy or HD cells, grown separately or as mosaics of the two genotypes. Single-cell RNA sequencing revealed neurodevelopmental abnormalities in the ventral fate acquisition of HD organoids, confirmed by cytoarchitectural and transcriptional defects leading to fewer GABAergic neurons, while dorsal populations showed milder phenotypes mainly in maturation trajectory. Healthy cells in mosaic organoids restored HD cell identity, trajectories, synaptic density, and communication pathways upon cell-cell contact, while showing no significant alterations when grown with HD cells. These findings highlight cell-type-specific alterations in HD and beneficial non-cell autonomous effects of healthy cells, emphasizing the therapeutic potential of modulating cell-cell communication in disease progression and treatment.
© 2024. The Author(s).
In Cell Death Dis on 22 April 2025 by Krzystek, T. J., Rathnayake, R., et al.
Fig.1.G

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 2
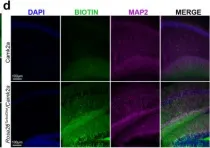
In Nat Commun on 25 May 2022 by Rayaprolu, S., Bitarafan, S., et al.
Fig.2.D

-
IHC-IF
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 2