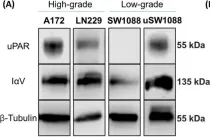
Integrin αV (IαV) and the urokinase-type plasminogen activator receptor (uPAR) are key mediators of tumor malignancy in Glioblastoma. This study aims to characterize IαV/uPAR interaction in GBM and investigate the role played by glycans in this scenario. Protein expression and interaction were confirmed via confocal microscopy and co-immunoprecipitation. The role of N-glycosylation was evaluated using Swainsonine (SW) and PNGase F. IαV glycoproteomic analysis was performed by mass spectrometry. Sialic acids and glycan structures in IαV/uPAR interaction were tested using neuraminidase A (NeuA) and lectin interference assays, respectively. Protein expression and their interaction were detected in GBM cells, but not in low-grade glioma cells, even in cells transfected to overexpress uPAR. SW, PNGase, and NeuA treatments significantly reduced IαV/uPAR interaction. Also, lectin interference assays indicated that β1-6 branched glycans play a crucial role in this interaction. Analysis of the IαV glycosylation profile revealed the presence of complex and hybrid N-glycans in GBM, while only oligomannose N-glycans were identified in low-grade glioma. N-glycosylation inhibition and sialic acid removal reduced AKT phosphorylation. Our findings demonstrate, for the first time, the interaction between IαV and uPAR in GBM cells, highlighting the essential role of N-glycosylation, particularly β1-6 branched glycans and sialic acids.
Product Citations: 65
In International Journal of Molecular Sciences on 31 May 2025 by Ferreira, G. M., Cuello, H., et al.
-
WB
-
Homo sapiens (Human)
In Journal of Experimental & Clinical Cancer Research : CR on 6 February 2025 by Rodrigues-Junior, D. M., Tsirigoti, C., et al.
Cancer cells are avid extracellular vesicle (EV) producers. EVs transport transforming growth factor-β (TGF-β), which is commonly activated under late stages of cancer progression. Nevertheless, whether TGF-β signaling coordinates EV biogenesis is a relevant topic that remains minimally explored.
We sought after specific TGF-β pathway mediators that could regulate EV release. To this end, we used a large number of cancer cell models, coupled to EV cell biological assays, unbiased proteomic and transcriptomic screens, followed by signaling and cancer biology analyses, including drug resistance assays.
We report that TGF-β, by activating its type I receptor and MEK-ERK1/2 signaling, increased the numbers of EVs released by human cancer cells. Upon examining cholesterol as a mediator of EV biogenesis, we delineated a pathway whereby ERK1/2 acted by phosphorylating sterol regulatory element-binding protein-2 that transcriptionally induced 7-dehydrocholesterol reductase expression, thus raising cholesterol abundance at both cellular and EV levels. Notably, inhibition of MEK or cholesterol synthesis, which impaired TGF-β-induced EV secretion, sensitized cancer cells to chemotherapeutic drugs. Furthermore, proteomic profiling of two distinct EV populations revealed that EVs secreted by TGF-β-stimulated cells were either depleted or enriched for different sets of cargo proteins. Among these, latent-TGF-β1 present in the EVs was not affected by TGF-β signaling, while TGF-β pathway-related molecules (e.g., matrix metalloproteinases, including MMP9) were either uniquely enriched on EVs or strongly enhanced after TGF-β stimulation. EV-associated latent-TGF-β1 activated SMAD signaling, even when EV uptake was blocked by heparin, indicating competent signaling capacity from target cell surface receptors. MMP inhibitor or proteinase treatment blocked EV-mediated SMAD signaling, suggesting that EVs require MMP activity to release the active TGF-β from its latent complex, a function also linked to the EV-mediated transfer of pro-migratory potential and ability of cancer cells to survive in the presence of cytotoxic drugs.
Hence, we delineated a novel signaling cascade that leads to high rates of EV generation by cancer cells in response to TGF-β, with cholesterol being a key intermediate step in this mechanism.
© 2025. The Author(s).
-
WB
-
Cancer Research
The Essential Role of N-Glycosylation in Integrin αV and uPAR Interaction in Glioblastoma
Preprint on Research Square on 2 October 2024 by Ferreira, G. M., Cuello, H. A., et al.
Abstract BACKGROUND Glioblastoma multiforme (GBM) is the most common and aggressive brain tumor in adults, characterized by poor patient survival rates. The glycoproteins Integrin αV (IαV), and the Urokinase-type plasminogen activator receptor (uPAR) are key contributors to tumor malignancy in GBM, and although their interaction is well-described, the role of glycans in this process has been scarcely evaluated. Better understanding this interaction could enhance our knowledge of the disease and lead to potential new therapeutics.METHODS We investigated the interaction between IαV and uPAR in human GBM, A172 and LN229, and low-grade glioma, SW1088, cell lines. Expression of these proteins was confirmed via confocal microscopy and co-immunoprecipitation. The role of N-glycosylation was evaluated using the inhibitor Swainsonine (SW) and glycosidase PNGase F. Glycoproteomic analysis by mass spectrometry identified glycosylation sites and differential structures on IαV. The impact of sialic acids and specific glycan structures was assessed using Neuraminidase (NeuA) and lectin binding assays.RESULTS The expression of IαV and uPAR, as well as their interaction, was confirmed in GBM cells but not in low-grade glioma cells, even when uPAR was overexpressed. SW and PNGase treatments markedly reduced IαV/uPAR interaction, highlighting the importance of N-glycosylation. Mass spectrometry analysis showed six glycosylation sites on IαV in GBM cells, with complex and hybrid N-glycans, while only oligomannose N-glycans were detected in low-grade glioma cells. NeuA treatment also reduced IαV/uPAR interaction, underscoring the role of sialic acids. Lectin assays suggested β1–6 branched glycans at specific sites are crucial for this interaction. Inhibition of N-glycosylation and sialic acid removal both decreased AKT phosphorylation, indicating a significant role of these glycans in integrin/uPAR signaling.CONCLUSIONS Our results demonstrate for the first time the interaction between IαV and uPAR in GBM cells, highlighting the critical role of N-glycosylation, particularly β1–6 branched glycans and sialic acids.
-
Homo sapiens (Human)
In International Journal of Biological Sciences on 21 June 2024 by Hu, K., Xie, L., et al.
Cysteine-rich angiogenic inducer 61 (CYR61), also called CCN1, has long been characterized as a secretory protein. Nevertheless, the intracellular function of CYR61 remains unclear. Here, we found that CYR61 is important for proper cell cycle progression. Specifically, CYR61 interacts with microtubules and promotes microtubule polymerization to ensure mitotic entry. Moreover, CYR61 interacts with PLK1 and accumulates during the mitotic process, followed by degradation as mitosis concludes. The proteolysis of CYR61 requires the PLK1 kinase activity, which directly phosphorylates two conserved motifs on CYR61, enhancing its interaction with the SCF E3 complex subunit FBW7 and mediating its degradation by the proteasome. Mutations of phosphorylation sites of Ser167 and Ser188 greatly increase CYR61's stability, while deletion of CYR61 extends prophase and metaphase and delays anaphase onset. In summary, our findings highlight the precise control of the intracellular CYR61 by the PLK1-FBW7 pathway, accentuating its significance as a microtubule-associated protein during mitotic progression.
© The author(s).
-
Homo sapiens (Human)
-
Cell Biology
In Cells on 6 February 2024 by Beckers, P., Doyen, P. J., et al.
Acting as GTPase activating proteins promoting the silencing of activated G-proteins, regulators of G protein signaling (RGSs) are generally considered negative modulators of cell signaling. In the CNS, the expression of RGS4 is altered in diverse pathologies and its upregulation was reported in astrocytes exposed to an inflammatory environment. In a model of cultured cortical astrocytes, we herein investigate the influence of RGS4 on intracellular calcium signaling mediated by type 5 metabotropic glutamate receptor (mGluR5), which is known to support the bidirectional communication between neurons and glial cells. RGS4 activity was manipulated by exposure to the inhibitor CCG 63802 or by infecting the cells with lentiviruses designed to achieve the silencing or overexpression of RGS4. The pharmacological inhibition or silencing of RGS4 resulted in a decrease in the percentage of cells responding to the mGluR5 agonist DHPG and in the proportion of cells showing typical calcium oscillations. Conversely, RGS4-lentivirus infection increased the percentage of cells showing calcium oscillations. While the physiological implication of cytosolic calcium oscillations in astrocytes is still under investigation, the fine-tuning of calcium signaling likely determines the coding of diverse biological events. Indirect signaling modulators such as RGS4 inhibitors, used in combination with receptor ligands, could pave the way for new therapeutic approaches for diverse neurological disorders with improved efficacy and selectivity.
-
WB
-
Cell Biology
-
Neuroscience
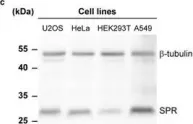
In Int J Mol Sci on 31 May 2025 by Ferreira, G. M., Cuello, H., et al.
Fig.1.A
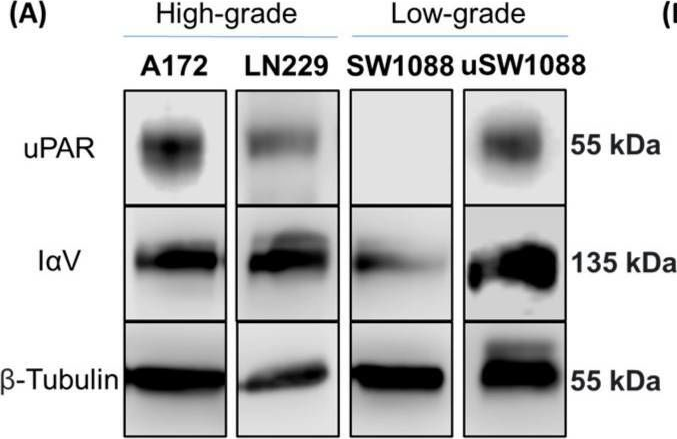
-
WB
-
Collected and cropped from International Journal of Molecular Sciences by CiteAb, provided under a CC-BY license
Image 1 of 9
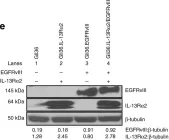
In Cancers (Basel) on 8 July 2020 by Ayo, A., Figueras, E., et al.
Fig.6.A
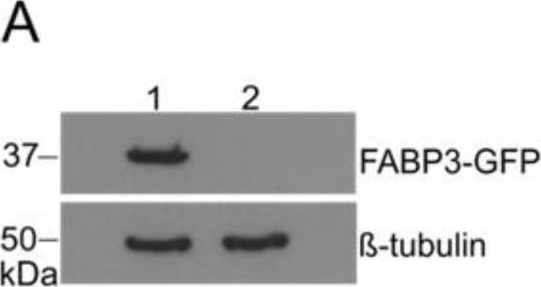
-
WB
-
Collected and cropped from Cancers by CiteAb, provided under a CC-BY license
Image 1 of 9
In PLoS One on 22 June 2018 by Yip, H. Y. K., Tan, C. W., et al.
Fig.6.B
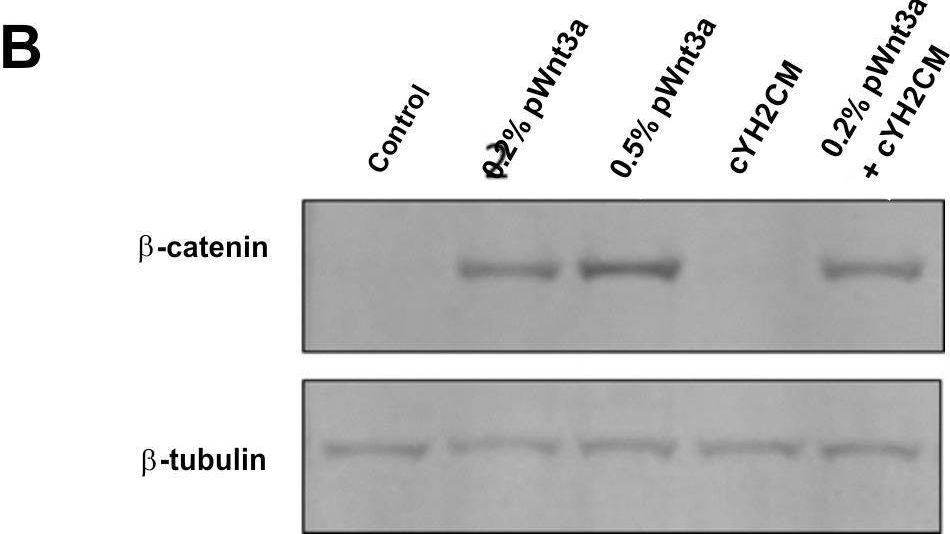
-
WB
-
Collected and cropped from PLoS ONE by CiteAb, provided under a CC-BY license
Image 1 of 9
In PLoS One on 22 June 2018 by Yip, H. Y. K., Tan, C. W., et al.
Fig.6.D
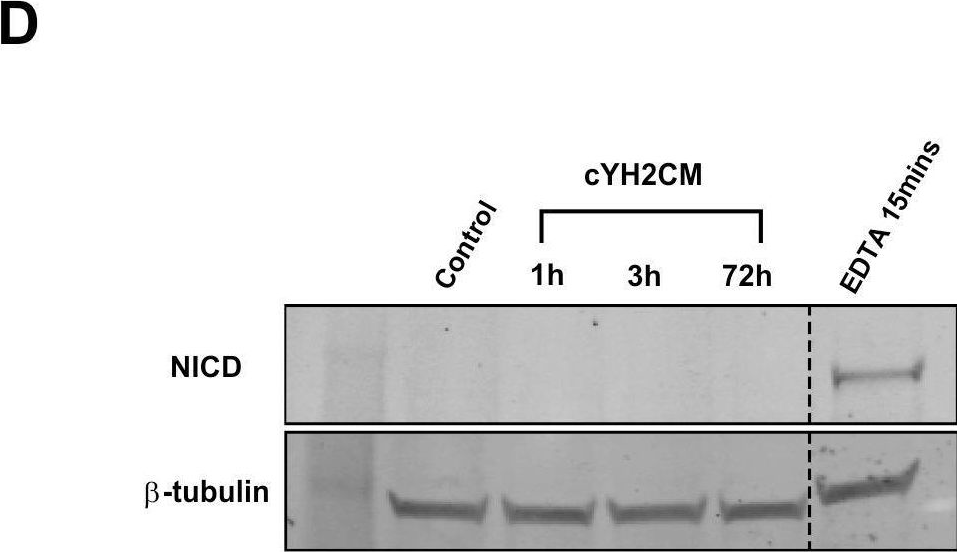
-
WB
-
Collected and cropped from PLoS ONE by CiteAb, provided under a CC-BY license
Image 1 of 9
In Elife on 29 May 2018 by Sallin, O., Reymond, L., et al.
Fig.3.C
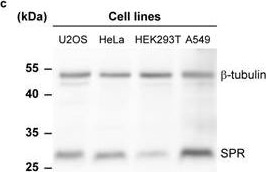
-
WB
-
Collected and cropped from eLife by CiteAb, provided under a CC-BY license
Image 1 of 9
In Nat Commun on 4 December 2017 by Newman, J. P., Wang, G. Y., et al.
Fig.1.E
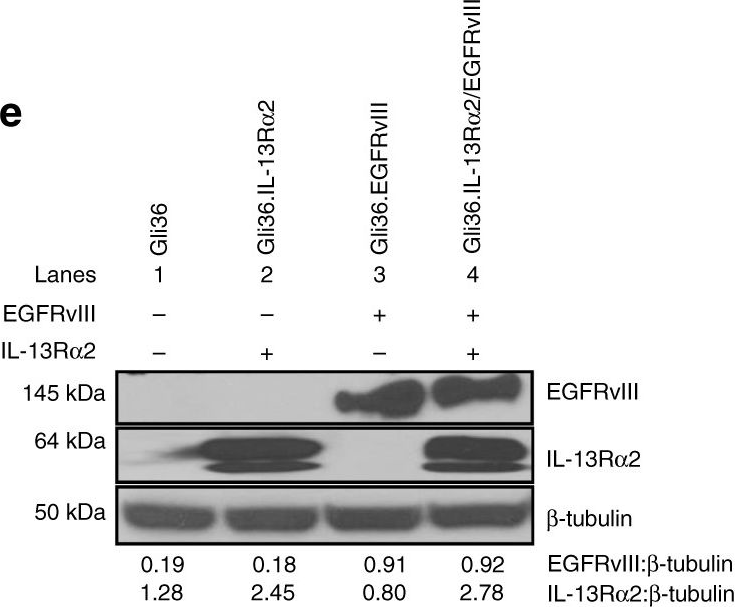
-
WB
-
Homo sapiens (Human)
Collected and cropped from Nature Communications by CiteAb, provided under a CC-BY license
Image 1 of 9
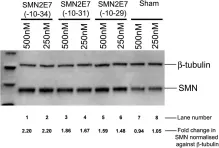
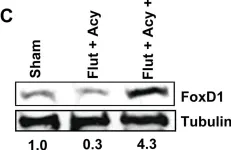
In PLoS One on 1 May 2013 by Mitrpant, C., Porensky, P., et al.
Fig.6.A
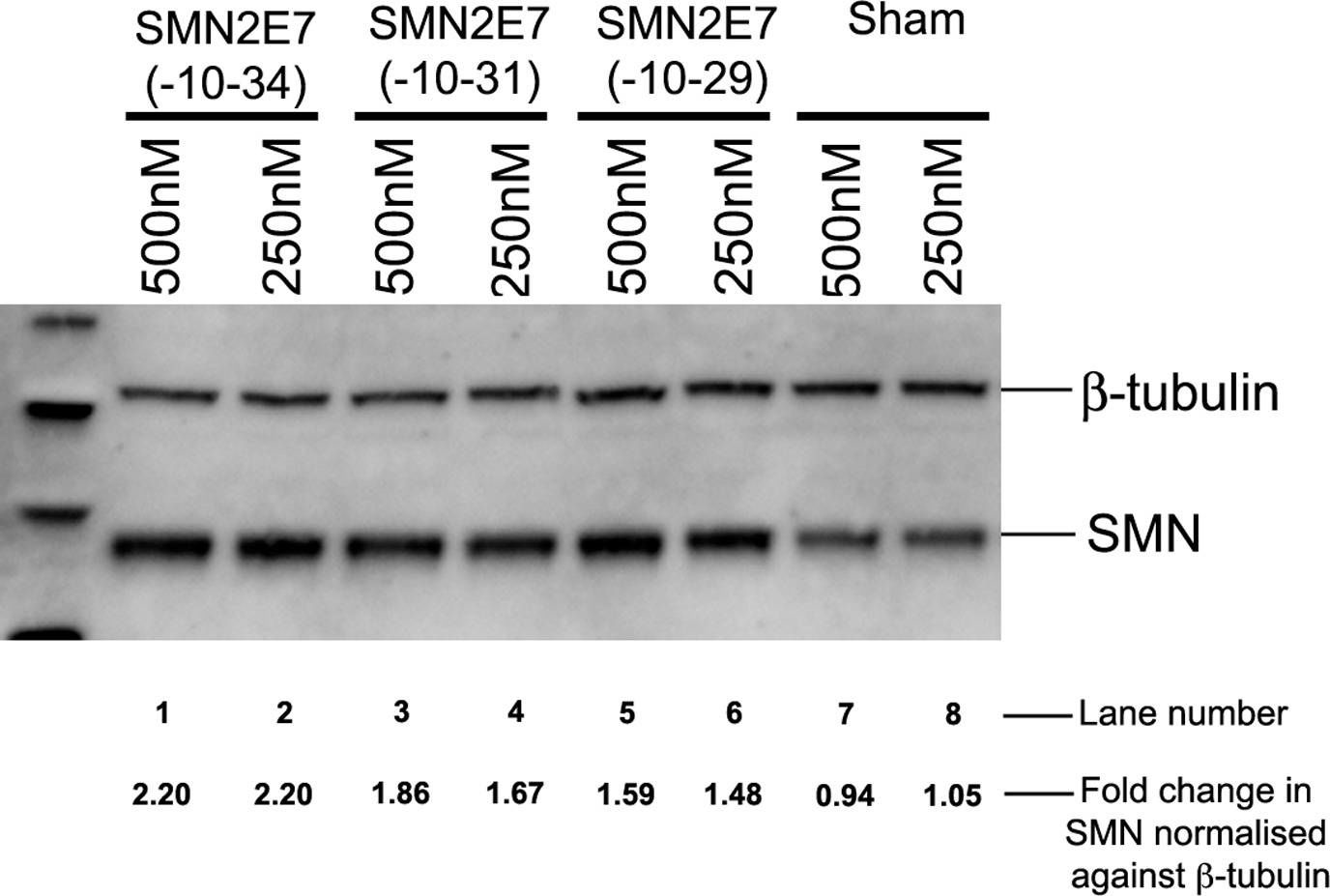
-
WB
-
Mus musculus (House mouse)
Collected and cropped from PLoS ONE by CiteAb, provided under a CC-BY license
Image 1 of 9
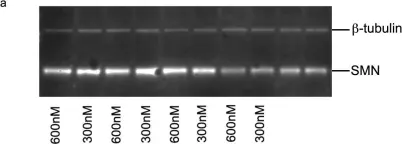
In PLoS One on 1 May 2013 by Mitrpant, C., Porensky, P., et al.
Fig.5.A
-
WB
-
Mus musculus (House mouse)
Collected and cropped from PLoS ONE by CiteAb, provided under a CC-BY license
Image 1 of 9
In PLoS One on 23 August 2012 by Panneerdoss, S., Chang, Y. F., et al.
Fig.6.C
-
WB
-
Mus musculus (House mouse)
Collected and cropped from PLoS ONE by CiteAb, provided under a CC-BY license
Image 1 of 9