Chronic use of selective serotonin reuptake inhibitors (SSRIs) for the treatment of depression has been linked to osteoporosis. In this study, we investigated the effect of chronic SSRI use on fracture healing in two murine models of bone regeneration. First, we performed a comprehensive analysis of endochondral bone healing in a femur fracture model. C57/BL6 mice treated with fluoxetine, the most commonly prescribed SSRI, developed a normal cartilaginous soft-callus at 14 days after fracture and demonstrated a significantly smaller and biomechanically weaker bony hard-callus at 28 days. In order to further dissect the mechanism that resulted in a smaller bony regenerate, we used an intramembranous model of bone healing and revealed that fluoxetine treatment resulted in a significantly smaller bony callus at 7 and 14 days postinjury. In order to test whether the smaller bony regenerate following fluoxetine treatment was caused by an inhibition of osteogenic differentiation and/or mineralization, we employed in vitro experiments, which established that fluoxetine treatment decreases osteogenic differentiation and mineralization and that this effect is serotonin-independent. Finally, in a translational approach, we tested whether cessation of the medication would result in restoration of the regenerative potential. However, histologic and μCT analysis revealed non-union formation in these animals with fibrous tissue interposition within the callus. In conclusion, fluoxetine exerts a direct, inhibitory effect on osteoblast differentiation and mineralization, shown in two disparate murine models of bone repair. Discontinuation of the drug did not result in restoration of the healing potential, but rather led to complete arrest of the repair process. Besides the well-established effect of SSRIs on bone homeostasis, our study provides strong evidence that fluoxetine use negatively impacts fracture healing. © 2017 American Society for Bone and Mineral Research.
© 2017 American Society for Bone and Mineral Research.
Product Citations: 8
In Journal of Bone and Mineral Research on 1 April 2017 by Bradaschia-Correa, V., Josephson, A. M., et al.
In Diabetologia on 1 April 2016 by Bennet, H., Mollet, I. G., et al.
The Gq-coupled 5-hydroxytryptamine 2B (5-HT2B) receptor is known to regulate the proliferation of islet beta cells during pregnancy. However, the role of serotonin in the control of insulin release is still controversial. The aim of the present study was to explore the role of the 5-HT2B receptor in the regulation of insulin secretion in mouse and human islets, as well as in clonal INS-1(832/13) cells.
Expression of HTR2B mRNA and 5-HT2B protein was examined with quantitative real-time PCR, RNA sequencing and immunohistochemistry. α-Methyl serotonin maleate salt (AMS), a serotonin receptor agonist, was employed for robust 5-HT2B receptor activation. Htr2b was silenced with small interfering RNA in INS-1(832/13) cells. Insulin secretion, Ca(2+) response and oxygen consumption rate were determined.
Immunohistochemistry revealed that 5-HT2B is expressed in human and mouse islet beta cells. Activation of 5-HT2B receptors by AMS enhanced glucose-stimulated insulin secretion (GSIS) in human and mouse islets as well as in INS-1(832/13) cells. Silencing Htr2b in INS-1(832/13) cells led to a 30% reduction in GSIS. 5-HT2B receptor activation produced robust, regular and sustained Ca(2+) oscillations in mouse islets with an increase in both peak distance (period) and time in the active phase as compared with control. Enhanced insulin secretion and Ca(2+) changes induced by AMS coincided with an increase in oxygen consumption in INS-1(832/13) cells.
Activation of 5-HT2B receptors stimulates GSIS in beta cells by triggering downstream changes in cellular Ca(2+) flux that enhance mitochondrial metabolism. Our findings suggest that serotonin and the 5-HT2B receptor stimulate insulin release.
-
WB
-
Endocrinology and Physiology
In Drug Development Research on 1 February 2015 by Pineda-Farias, J. B., Velazquez-Lagunas, I., et al.
Preclinical Research This work was performed to assess the effects of intrathecal serotonin 2B (5-HT2B ) receptor antagonists in rats with neuropathic pain. With RS-127445, its effect was also determined on 5-HT2B receptor expression. Neuropathic pain was induced by L5/L6 spinal nerve ligation. Western blotting was used to determine 5-HT2B receptor expression. Dose-response curves with the 5-HT2B receptor antagonists 2-amino-4-(4-fluoronaphth-1-yl)-6-isopropylpyridine (RS-127445, 1-100 nmol) and 1-[(2-chloro-3,4-dimethoxyphenyl)methyl]-2,3,4,9-tetrahydro-6-methyl-1H-pyrido[3,4-b]indole hydrochloride (LY-266097, 1-100 nmol) were performed in rats. Tactile allodynia of the left hind paw (ipsilateral) was assessed for 8 h after compound administration. Intrathecal injection of the 5-HT2B receptor antagonists RS-127445 and LY-266097 diminished spinal nerve ligation-induced allodynia. In contrast, intrathecal injection of the 5-HT2 receptor agonist (±)-2,5-dimethoxy-4-iodoamphetamine hydrochloride (DOI, 10 nmol) did not modify tactile allodynia induced by nerve ligation. L5/L6 nerve ligation increased expression of the 5-HT2B receptors in the ipsilateral, but not contralateral, dorsal root ganglia. Furthermore, nerve injury also enhanced 5-HT2B receptor expression in the ipsilateral dorsal part of the spinal cord. Intrathecal treatment with RS-127445 (100 nmol) diminished spinal nerve injury-induced increased expression of 5-HT2B receptors in dorsal root ganglia and spinal cord. Our results imply that spinal 5-HT2B receptors are present on sites related to nociception and participate in neuropathic pain. © 2014 Wiley Periodicals, Inc.
© 2014 Wiley Periodicals, Inc.
-
Neuroscience
5-HT is a potent relaxant in rat superior mesenteric veins.
In Pharmacology Research Perspectives on 1 February 2015 by Watts, S. W., Darios, E. S., et al.
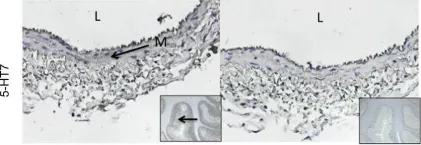
Serotonin (5-HT, 5-hydroxytryptamine) reduces blood pressure of the conscious rat when administered chronically (1 week). 5-HT does not directly relax isolated arteries, and microsphere experiments in 5-HT-infused rats suggested that 5-HT increased flow to the splanchnic bed. We hypothesized that 5-HT increased splanchnic flow because of direct venous relaxation; our focus was thus on the superior mesenteric vein (SMV) as an important vein in splanchnic circulation. Real-time RT-PCR, immunohistochemistry and Western analyses supported the predominant expression of the 5-HT2B and 5-HT7 receptor in the SMV. The SMV was mounted in tissue baths for measurement of isometric contraction. 5-HT caused a concentration-dependent relaxation of the endothelin-1 (ET-1)-contracted vein. The threshold of 5-HT-induced venous relaxation was significantly lower than for 5-HT-induced venous contraction (∼2 vs. 700 nmol/L, respectively). A series of serotonergic agonists established in their use of receptor characterization was tested, and the following rank order of potency found for agonist-induced relaxation (receptor selectivity): 5-CT (5-HT1/5-HT7)>5-HT = LP-44 (5-HT7)>PNU109291 (5-HT1D) = BW723C86 (5-HT2B). 8-OH-DPAT (5-HT1A/7), CP93129 (5-HT1B), mCPBG (5-HT3/4), AS19 (5-HT7) and TCB-2 (5-HT2A) did not relax the isolated vein. Consistent with these findings, two different 5-HT7 receptor antagonists SB 269970 and LY215840 but not the 5-HT2B receptor antagonist LY272015 nor the nitric oxide synthase inhibitor LNNA abolished 5-CT-induced relaxation of the isolated SMV. 5-CT (1 μg kg(-1) min(-1), sc) also reduced blood pressure over 7 days. These findings suggest that 5-HT directly relaxes the SMV primarily through activation of the 5-HT7 receptor.
-
IHC
-
WB
-
Rattus norvegicus (Rat)
-
Pharmacology
5HT(2A) and 5HT(2B) receptors contribute to serotonin-induced vascular dysfunction in diabetes.
In Experimental Diabetes Research on 25 January 2013 by Nelson, P. M., Harrod, J. S., et al.
Although 5HT(2A) receptors mediate contractions of normal arteries to serotonin (5HT), in some cardiovascular diseases, other receptor subtypes contribute to the marked increase in serotonin contractions. We hypothesized that enhanced contractions of arteries from diabetics to 5HT are mediated by an increased contribution from multiple 5HT receptor subtypes. We compared responses to selective 5HT receptor agonists and expression of 5HT receptor isoforms (5HT(1B), 5HT(2A), and 5HT(2B)) in aorta from nondiabetic (ND) compared to type 2 diabetic mice (DB, BKS.Cg-Dock7(m)+/+Lepr(db)/J). 5HT, 5HT(2A) (TCB2 and BRL54443), and 5HT(2B) (norfenfluramine and BW723C86) receptor agonists produced concentration-dependent contractions of ND arteries that were markedly increased in DB arteries. Neither ND nor DB arteries contracted to a 5HT(1B) receptor agonist. MDL11939, a 5HT(2A) receptor antagonist, and LY272015, a 5HT(2B) receptor antagonist, reduced contractions of arteries from DB to 5HT more than ND. Expression of 5HT(1B), 5HT(2A), and 5HT(2B) receptor subtypes was similar in ND and DB. Inhibition of rho kinase decreased contractions to 5HT and 5HT(2A) and 5HT(2B) receptor agonists in ND and DB. We conclude that in contrast to other cardiovascular diseases, enhanced contraction of arteries from diabetics to 5HT is not due to a change in expression of multiple 5HT receptor subtypes.
-
WB
In Pharmacol Res Perspect on 1 February 2015 by Watts, S. W., Darios, E. S., et al.
Fig.2.A

-
IHC
-
Rattus norvegicus (Rat)
Collected and cropped from Pharmacol Res Perspect by CiteAb, provided under a CC-BY license
Image 1 of 1