Diffuse large B cell lymphomas (DLBCL) are a highly heterogeneous subtype of Non Hodgkin Lymphoma (NHL), accounting for about 25% of NHL. Despite an increased progression-free survival upon therapy, 40-50% of patients develop relapse/refractory disease, therefore there remains an important medical need. T cell recruiting therapies, such as the CD20xCD3 T cell bi-specific antibody CD20-TCB (RG6026 or glofitamab), represent a novel approach to target all stages of DLBCL, especially those that fail to respond to multiple lines of treatment. We aimed for a better understanding of the molecular features related to the mode of action (MoA) of CD20-TCB in inducing Target/T cell synapse formation and human T cell recruitment to the tumor. To directly evaluate the correlation between synapse, cytokine production and anti-tumor efficacy using CD20-TCB, we developed an innovative preclinical human DLBCL in vivo model that allowed tracking in vivo human T cell dynamics by multiphoton intravital microscopy (MP-IVM). By ex vivo and in vivo approaches, we revealed that CD20-TCB is inducing strong and stable synapses between human T cell and tumor cells, which are dependent on the dose of CD20-TCB and on LFA-1 activity but not on FAS-L. Moreover, despite CD20-TCB being a large molecule (194.342 kDa), we observed that intra-tumor CD20-TCB-mediated human T cell-tumor cell synapses occur within 1 hour upon CD20-TCB administration. These tight interactions, observed for at least 72 hours post TCB administration, result in tumor cell cytotoxicity, resident T cell proliferation and peripheral blood T cell recruitment into tumor. By blocking the IFNγ-CXCL10 axis, the recruitment of peripheral T cells was abrogated, partially affecting the efficacy of CD20-TCB treatment which rely only on resident T cell proliferation. Altogether these data reveal that CD20-TCB's anti-tumor activity relies on a triple effect: i) fast formation of stable T cell-tumor cell synapses which induce tumor cytotoxicity and cytokine production, ii) resident T cell proliferation and iii) recruitment of fresh peripheral T cells to the tumor core to allow a positive enhancement of the anti-tumor effect.
Product Citations: 62
In PLoS ONE on 7 January 2021 by Cremasco, F., Menietti, E., et al.
-
Homo sapiens (Human)
-
Cancer Research
-
Immunology and Microbiology
In Cancer Science on 1 March 2020 by Masuda, A., Isobe, Y., et al.
Activation-induced cell death (AICD) mediated by the Fas/Fas ligand (FasL) system plays a key role in regulating immune response. Although normal natural killer (NK) cells use this system for their homeostasis, malignant NK cells seem to disrupt the process. Extranodal NK/T-cell lymphoma, nasal type (ENKL) is a rare but fatal disease, for which novel therapeutic targets need to be identified. We confirmed that ENKL-derived NK cell lines NK-YS and Hank1, and primary lymphoma cells expressed procaspase-8/FADD-like interleukin-1β-converting enzyme (FLICE) modulator and cellular FLICE-inhibitory protein (c-FLIP), along with Fas and FasL. Compared with Fas-sensitive Jurkat cells, NK-YS and Hank1 showed resistance to Fas-mediated apoptosis in spite of the same expression levels of c-FLIP and the death-inducing signaling complex (DISC) formation. Unexpectedly, the long isoform of c-FLIP (c-FLIPL ) was coimmunoprecipitated with Fas predominantly in both ENKL-derived NK cell lines after Fas ligation. Indeed, c-FLIPL was more sufficiently recruited to the DISC in both ENKL-derived NK cell lines than in Jurkat cells after Fas ligation. Knockdown of c-FLIPL per se enhanced autonomous cell death and restored the sensitivity to Fas in both NK-YS and Hank1 cells. Although ENKL cells are primed for AICD, they constitutively express and efficiently utilize c-FLIPL , which prevents their Fas-mediated apoptosis. Our results show that c-FLIPL could be a promising therapeutic target against ENKL.
© 2020 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
-
Cancer Research
Fatty Acid Synthase Contributes to Restimulation-Induced Cell Death of Human CD4 T Cells.
In Frontiers in Molecular Biosciences on 5 November 2019 by Voss, K., Luthers, C. R., et al.
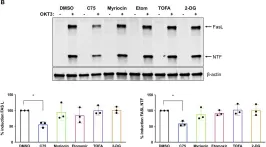
Restimulation-induced cell death (RICD) is an apoptotic pathway triggered in activated effector T cells after T cell receptor (TCR) re-engagement. RICD operates at the peak of the immune response to ensure T cell expansion remains in check to maintain immune homeostasis. Understanding the biochemical regulation of RICD sensitivity may provide strategies for tuning the magnitude of an effector T cell response. Metabolic reprogramming in activated T cells is not only critical for T cell differentiation and effector functions, but also influences apoptosis sensitivity. We previously demonstrated that aerobic glycolysis correlates with optimum RICD sensitivity in human effector CD8 T cells. However, metabolic programming in CD4 T cells has not been investigated in this context. We employed a pharmacological approach to explore the effects of fatty acid and glycolytic metabolism on RICD sensitivity in primary human CD4 T cells. Blockade of fatty acid synthase (FASN) with the compound C75 significantly protected CD4 effector T cells from RICD, suggesting that fatty acid biosynthesis contributes to RICD sensitivity. Interestingly, sphingolipid synthesis and fatty acid oxidation (FAO) were dispensable for RICD. Disruption of glycolysis did not protect CD4 T cells from RICD unless glyceraldehyde-3-phosphate dehydrogenase (GAPDH) enzymatic activity was targeted specifically, highlighting important differences in the metabolic control of RICD in effector CD4 vs. CD8 T cell populations. Moreover, C75 treatment protected effector CD4 T cells derived from naïve, effector memory, and central memory T cell subsets. Decreased RICD in C75-treated CD4 T cells correlated with markedly reduced FAS ligand (FASL) induction and a Th2-skewed phenotype, consistent with RICD-resistant CD4 T cells. These findings highlight FASN as a critical metabolic potentiator of RICD in human effector CD4 T cells.
Copyright © 2019 Voss, Luthers, Pohida and Snow.
-
WB
-
Immunology and Microbiology
In Journal of Applied Toxicology : JAT on 1 March 2019 by Guerrero-Palomo, G., Rendón-Huerta, E. P., et al.
Non-small lung cell carcinoma has a high morbidity and mortality rates. The elective treatment for stage III and IV is cisplatinum that conveys serious toxic side effects. Vanadium compounds are metal molecules with proven antitumor activity that depends on its valence. Therefore, a better understanding of the mechanism of action of vanadium compounds is required. The aim of our study was to investigate the mechanisms of cell death induced by sodium metavanadate (NaVO3 [V(+5)]) and vanadyl sulfate (VOSO4 [(+4)]), both of which have reported apoptotic-inducing activity. We exposed the A549 cell line to various concentrations (0-100 μM) and to different exposure times to each compound and determined the cell viability and expression of caspases, reactive oxygen species (ROS) production, Bcl2, Bax, FasL and NO. Our results showed that neither compounds modified the basal expression of caspases or pro- and anti-apoptotic proteins. The only change observed was the 12- and 14-fold significant increase in ROS production induced by NaVO3 and VOSO4 , respectively, at 100 μm concentrations after 48 hours. Our results suggest that classical apoptotic mechanisms are not related to the cell death induced by the vanadium compounds evaluated here, and showed that the higher ROS production was induced by the [(+4)] valence compound. It is possible that the difference will be secondary to its higher oxidative status and thus higher ROS production, which leads to higher cell damage. In conclusion, our results suggest that the efficacy of the cell death mechanisms induced by vanadium compounds differ depending on the valence of the compound.
© 2018 John Wiley & Sons, Ltd.
In Cell Death & Disease on 22 January 2018 by Glukhova, X. A., Trizna, J. A., et al.
Fas-ligand/CD178 belongs to the TNF family proteins and can induce apoptosis through death receptor Fas/CD95. The important requirement for Fas-ligand-dependent cell death induction is its localization to rafts, cholesterol- and sphingolipid-enriched micro-domains of membrane, involved in regulation of different signaling complexes. Here, we demonstrate that Fas-ligand physically associates with caveolin-1, the main protein component of rafts. Experiments with cells overexpressing Fas-ligand revealed a FasL N-terminal pre-prolin-rich region, which is essential for the association with caveolin-1. We found that the N-terminal domain of Fas-ligand bears two caveolin-binding sites. The first caveolin-binding site binds the N-terminal domain of caveolin-1, whereas the second one appears to interact with the C-terminal domain of caveolin-1. The deletion of both caveolin-binding sites in Fas-ligand impairs its distribution between cellular membranes, and attenuates a Fas-ligand-induced cytotoxicity. These results demonstrate that the interaction of Fas-ligand and caveolin-1 represents a molecular basis for Fas-ligand translocation to rafts, and the subsequent induction of Fas-ligand-dependent cell death. A possibility of a similar association between other TNF family members and caveolin-1 is discussed.
-
Cell Biology
In Front Mol Biosci on 5 November 2019 by Voss, K., Luthers, C. R., et al.
Fig.6.B

-
WB
-
Collected and cropped from Front Mol Biosci by CiteAb, provided under a CC-BY license
Image 1 of 1