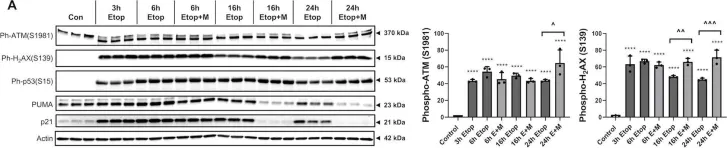
Mammalian cells entering the cell cycle favour glycolysis to rapidly generate ATP and produce the biosynthetic intermediates that are required for rapid biomass accumulation1. Simultaneously, the ubiquitin-ligase anaphase-promoting complex/cyclosome and its coactivator CDH1 (APC/CCDH1) remains active, allowing origin licensing and blocking premature DNA replication. Paradoxically, glycolysis is reduced by APC/CCDH1 through the degradation of key glycolytic enzymes2, raising the question of how cells coordinate these mutually exclusive events to ensure proper cell division. Here we show that cells resolve this paradox by transiently inactivating the APC/C during cell cycle entry, which allows a transient metabolic shift favouring glycolysis. After mitogen stimulation, rapid mTOR-mediated phosphorylation of the APC/C adapter protein CDH1 at the amino terminus causes it to partially dissociate from the APC/C. This partial inactivation of the APC/C leads to the accumulation of PFKFB3, a rate-limiting enzyme for glycolysis, promoting a metabolic shift towards glycolysis. Delayed accumulation of phosphatase activity later removes CDH1 phosphorylation, restoring full APC/C activity, and shifting cells back to favouring oxidative phosphorylation. Thus, cells coordinate the simultaneous demands of cell cycle progression and metabolism through an incoherent feedforward loop, which transiently inhibits APC/C activity to generate a pulse of glycolysis that is required for mammalian cell cycle entry.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Product Citations: 265
Transient APC/C inactivation by mTOR boosts glycolysis during cell cycle entry.
In Nature on 1 October 2025 by Paul, D., Bolhuis, D. L., et al.
In Aging Cell on 1 September 2025 by Liu, K., Fan, D., et al.
Microglia-mediated neuroinflammation has been shown to exert an important effect on the progression of a growing number of neurodegenerative disorders. Prolonged exposure to detrimental stimuli leads to a state of progressive activation and aging-related features in microglia (also termed as senescent microglia). However, the mechanisms by which senescent microglia contribute to neuroinflammation-induced cognitive dysfunction remain to be elucidated. Here, we developed a mouse model of neuroinflammation induced by lipopolysaccharides at 0.5 mg/kg for 7 consecutive days. To evaluate cognitive function, C57BL/6J mice were employed and subjected to a series of behavioral assessments, including the open field, Y-maze, and novel object recognition tests. Employing single-cell RNA sequencing technology, we have delved into the differential expressions of RNA within microglia. Furthermore, to investigate anatomic and physiological alterations of pyramidal neurons, we utilized Golgi staining and whole-cell patch-clamp recordings, respectively. Validation of our results in protein expression was performed using western blotting and immunofluorescence. We specifically identified senescent microglia with a high expression of p16INK4a and observed that microglia in the hippocampal CA1 region of the model exhibited signatures of elevated phagocytosis and senescence. A senolytic by ABT-737 treatment alleviated the production of senescence-associated secretory phenotypes, the accumulation of senescent microglia, and the microglial hyperphagocytosis of excitatory synapses following LPS exposures. This treatment also restored reduced excitatory synaptic transmission, impaired long-term potentiation, and cognitive function in the model. These results indicate that reducing senescent microglia may potentially serve as a therapeutic approach to prevent neuroinflammation-related cognitive dysfunction.
© 2025 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
-
Neuroscience
In GeroScience on 1 April 2025 by Hung, Y. L., Sato, A., et al.
Accumulation of senescent cells in tissues contributes to multiple aging-related pathologies. Senescent fibro-adipogenic progenitors (FAPs) contribute to aging-related muscle atrophy. Resistance training can help to maintain skeletal muscle mass, improve mobility, and reduce certain health risks commonly associated with aging. We investigated, using rat model, the impact of resistance training on FAPs in aging skeletal muscle, which remains unclear. Twenty-two-month-old female rats were divided into sedentary and training groups. The training group rodents were trained to climb a ladder while bearing a load for 20 training sessions over 2 months, after which, the flexor hallucis longus muscles were collected and analyzed. Senescent cells were identified using a senescence-associated β-galactosidase stain and p21 immunohistochemistry (IHC), and FAPs were identified using platelet-derived growth factor receptor alpha IHC. The results indicate that resistance training in rats prevented aging-associated skeletal muscle atrophy and suppressed M2 polarization of macrophages. The number of senescent cells was significantly reduced in the 24-month-old training group, with most of them being FAPs. Conversely, the number of senescent FAPs increased significantly in the 24-month-old sedentary group compared with that in the 18-month-old sedentary group. The number of senescent FAPs in the 24-month-old training group decreased significantly. Resistance training also suppressed the senescence-associated secretory phenotype (SASP). The killer T cell-specific marker, CD8α, was elevated in the skeletal muscles of the aging rats following resistance training, indicating upregulation of recognition and elimination of senescent cells. Overall, resistance training suppressed the accumulation of senescent FAPs and acquisition of SASP in aging skeletal muscles.
© 2024. The Author(s).
-
IHC
-
Rattus norvegicus (Rat)
In Small (Weinheim An Der Bergstrasse, Germany) on 1 February 2025 by Parshad, B., Baker, A. G., et al.
Cellular senescence has recently been recognized as one of the hallmarks of cancer, aging, as well as many age-related disorders, sparking significant interest in the development of senolytics, compounds that can remove senescent cells. However, most current pharmacological strategies face challenges related to non-specific delivery, leading to significant side effects that hinder safe and effective treatments. To address these issues, galactose-functionalized amphiphiles are synthesized that can self-assemble into micelles and be loaded with a senolytic cargo. These galactose-micelles are responsive to the lysosomal β-galactosidase enzyme, present in elevated amounts in senescent cells, and are employed for specific delivery of the senolytic Bcl2-inhibitor Navitoclax. This novel formulation showed reduced delivery and toxicity to non-senescent cells, thereby increasing the senolytic index of Navitoclax and making it suitable for future in vivo experimental designs to improve selectivity and safety profiles.
© 2024 The Author(s). Small published by Wiley‐VCH GmbH.
In Cell Death & Disease on 26 January 2025 by Yanushko, D., German, B., et al.
Prostate cancer is a heterogeneous disease with a slow progression and a highly variable clinical outcome. The tumor suppressor genes PTEN and TP53 are frequently mutated in prostate cancer and are predictive of early metastatic dissemination and unfavorable patient outcomes. The progression of solid tumors to metastasis is often associated with increased cell plasticity, but the complex events underlying TP53-loss-induced disease aggressiveness remain incompletely understood. Using genetically engineered mice, we show that Trp53 deficiency in Pten-null prostatic epithelial cells (PECs) does not impact early cell proliferation and neoplasia formation, nor growth arrest and senescence entry at a later time. However, Trp53-deficiency enhances invasive adenocarcinoma development and promotes metastatic cell dissemination. Importantly, our single-cell transcriptomic and chromatin accessibility analyses combined with histological examinations uncovered an epithelial cell population characterized by an induction of Jak/Stat3 signaling and displaying mesenchymal features. Moreover, we show that the transcriptomic signature of this cell population is prominent in tumors of patients with high-risk prostate cancer or metastatic disease. In addition, our in vivo and organoid-based experiments provide evidence that PEC plasticity occurs through bi-directional communication with cancer-associated fibroblasts (CAFs). Thus, our study demonstrates that p53 loss induces a protumorigenic crosstalk between PECs and CAFs, and identifies new vulnerabilities that might be targeted to limit cancer progression.
© 2025. The Author(s).
-
IHC
-
Mus musculus (House mouse)
-
Cancer Research
-
Cell Biology
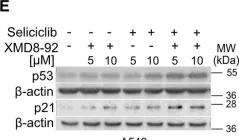
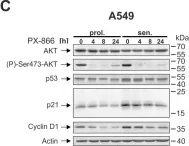
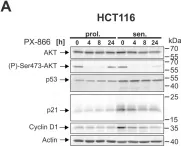
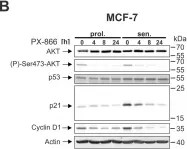
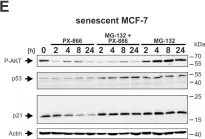
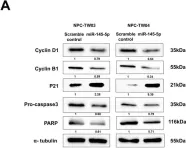
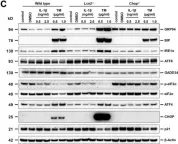
In EMBO Mol Med on 1 October 2024 by Pozzato, C., Outeiro-Pinho, G., et al.
Fig.2.E
-
WB
-
Collected and cropped from EMBO Molecular Medicine by CiteAb, provided under a CC-BY license
Image 1 of 70
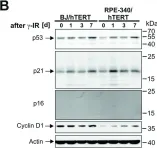
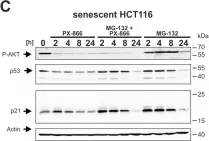
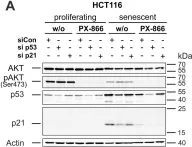
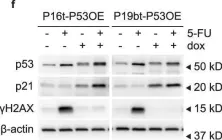
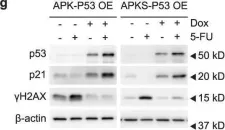
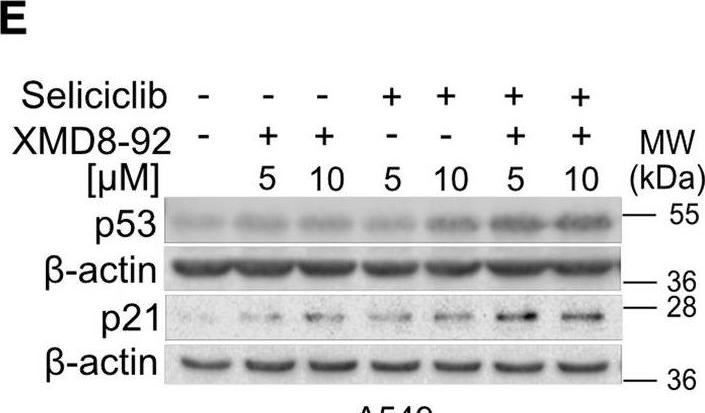
In Cell Death Dis on 29 May 2024 by Neuwahl, J., Neumann, C. A., et al.
Fig.8.B
-
WB
-
Collected and cropped from Cell Death & Disease by CiteAb, provided under a CC-BY license
Image 1 of 70
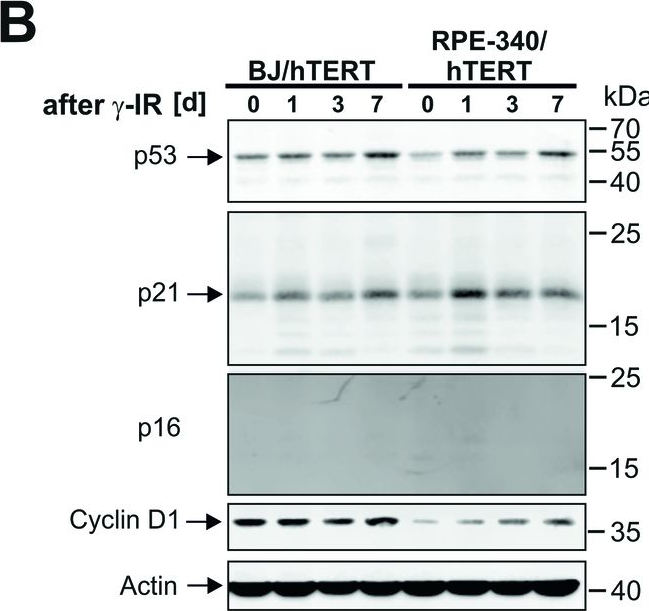
In Cell Death Dis on 29 May 2024 by Neuwahl, J., Neumann, C. A., et al.
Fig.6.C
-
WB
-
Collected and cropped from Cell Death & Disease by CiteAb, provided under a CC-BY license
Image 1 of 70
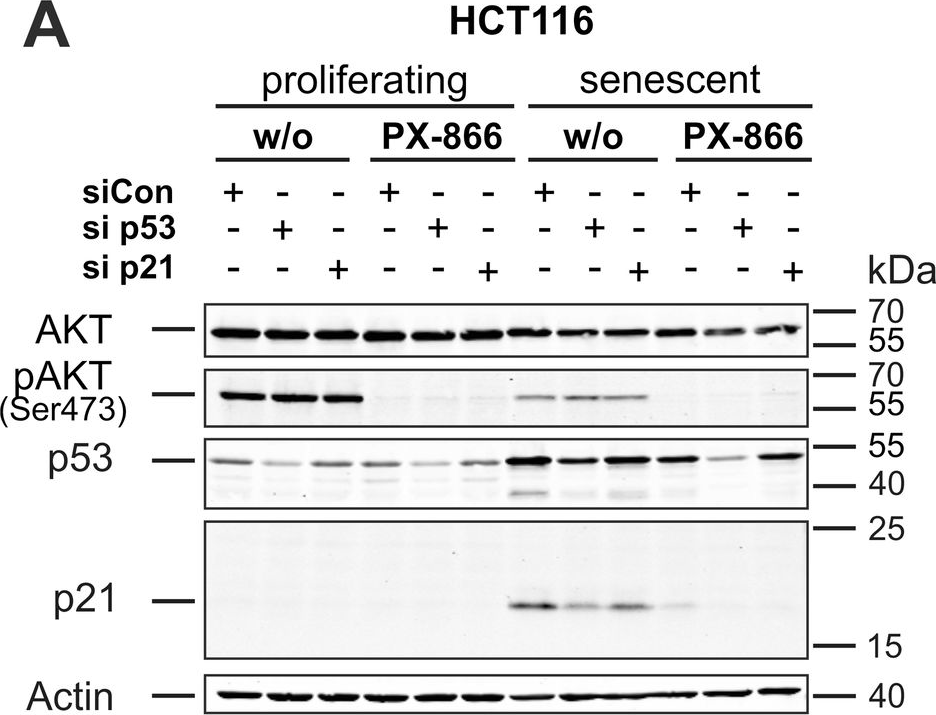
In Cell Death Dis on 29 May 2024 by Neuwahl, J., Neumann, C. A., et al.
Fig.6.A
-
WB
-
Collected and cropped from Cell Death & Disease by CiteAb, provided under a CC-BY license
Image 1 of 70
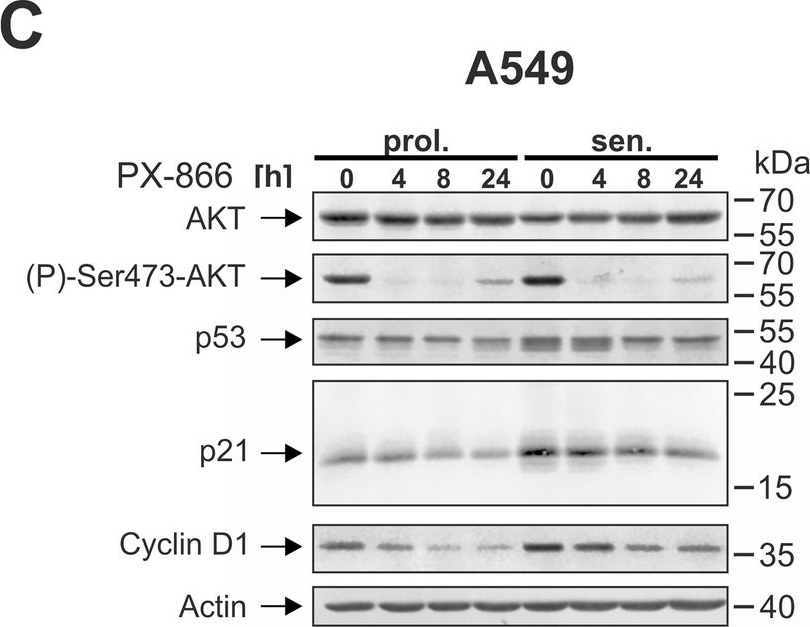
In Cell Death Dis on 29 May 2024 by Neuwahl, J., Neumann, C. A., et al.
Fig.5.C
-
WB
-
Collected and cropped from Cell Death & Disease by CiteAb, provided under a CC-BY license
Image 1 of 70
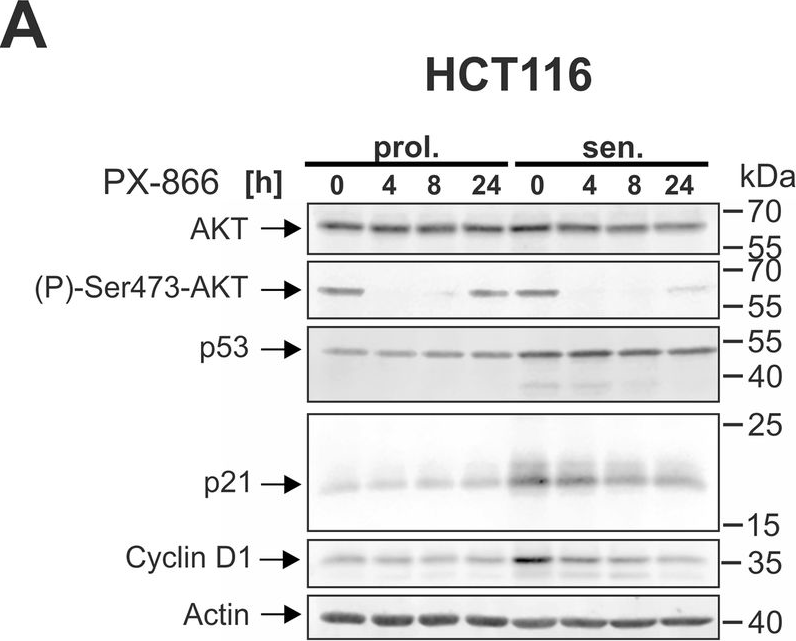
In Cell Death Dis on 29 May 2024 by Neuwahl, J., Neumann, C. A., et al.
Fig.5.A
-
WB
-
Collected and cropped from Cell Death & Disease by CiteAb, provided under a CC-BY license
Image 1 of 70
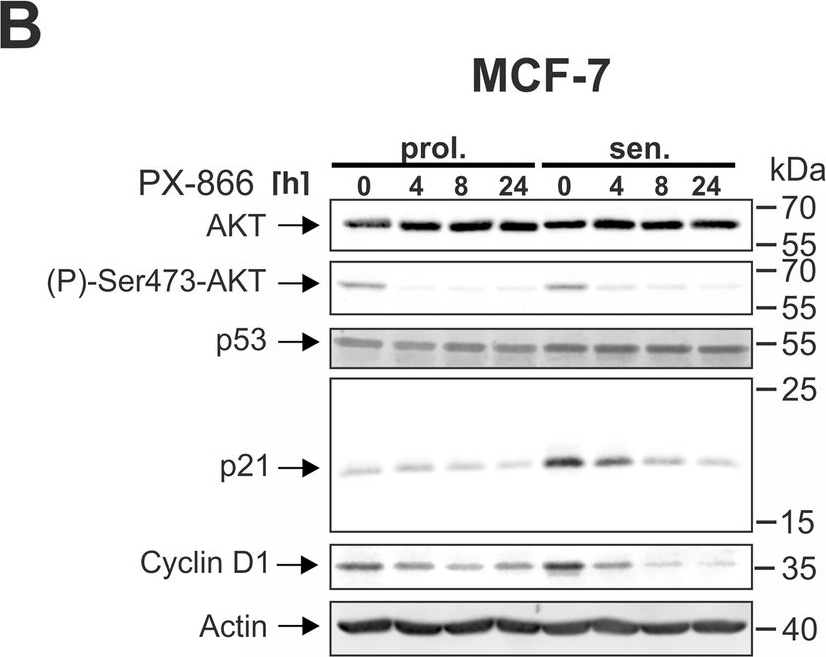
In Cell Death Dis on 29 May 2024 by Neuwahl, J., Neumann, C. A., et al.
Fig.5.B
-
WB
-
Collected and cropped from Cell Death & Disease by CiteAb, provided under a CC-BY license
Image 1 of 70
In Cell Death Dis on 29 May 2024 by Neuwahl, J., Neumann, C. A., et al.
Fig.6.E
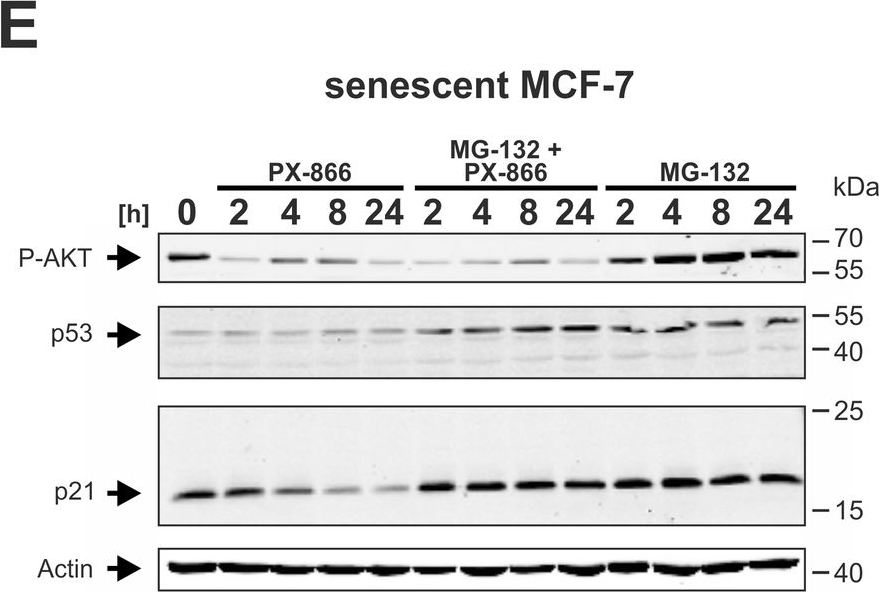
-
WB
-
Collected and cropped from Cell Death & Disease by CiteAb, provided under a CC-BY license
Image 1 of 70
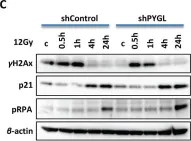
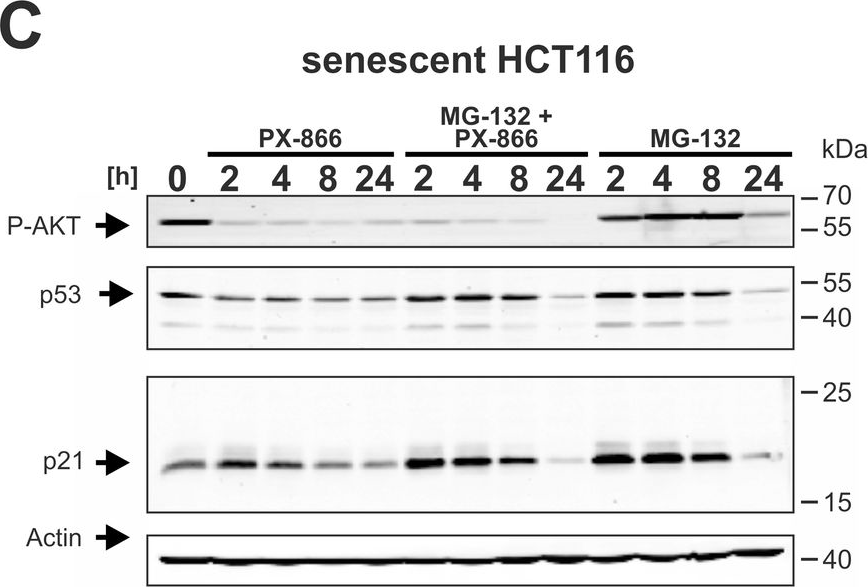
In J Cell Mol Med on 1 September 2023 by Vini, R., Lekshmi, A., et al.
Fig.2.F
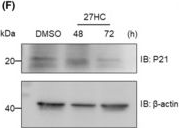
-
WB
-
Homo sapiens (Human)
Collected and cropped from Journal of Cellular and Molecular Medicine by CiteAb, provided under a CC-BY license
Image 1 of 70
In Front Neurosci on 22 November 2022 by Schwab, N., Taskina, D., et al.
Fig.7.A
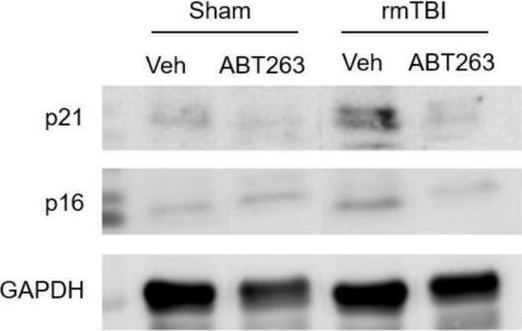
-
WB
-
Mus musculus (House mouse)
Collected and cropped from Frontiers in Neuroscience by CiteAb, provided under a CC-BY license
Image 1 of 70
In Front Neurosci on 22 November 2022 by Schwab, N., Taskina, D., et al.
Fig.7.C
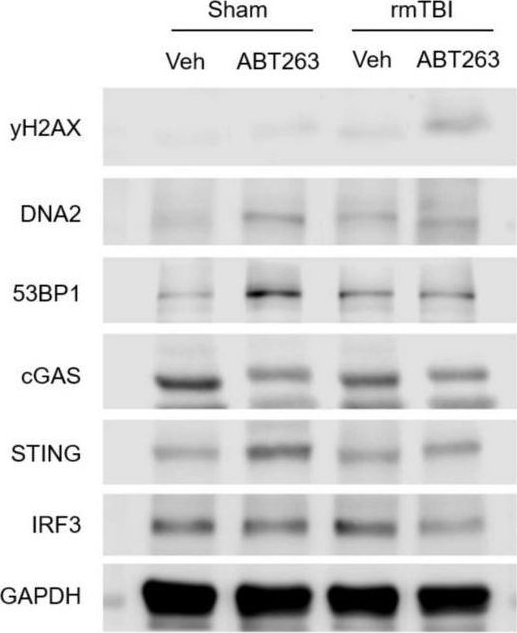
-
WB
-
Mus musculus (House mouse)
Collected and cropped from Frontiers in Neuroscience by CiteAb, provided under a CC-BY license
Image 1 of 70
In Front Neurosci on 22 November 2022 by Schwab, N., Taskina, D., et al.
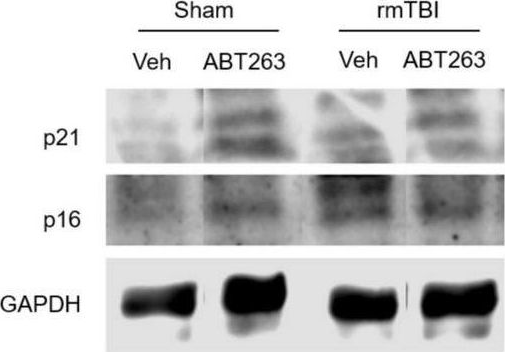
Fig.7.B
-
WB
-
Mus musculus (House mouse)
Collected and cropped from Frontiers in Neuroscience by CiteAb, provided under a CC-BY license
Image 1 of 70
In Front Neurosci on 22 November 2022 by Schwab, N., Taskina, D., et al.
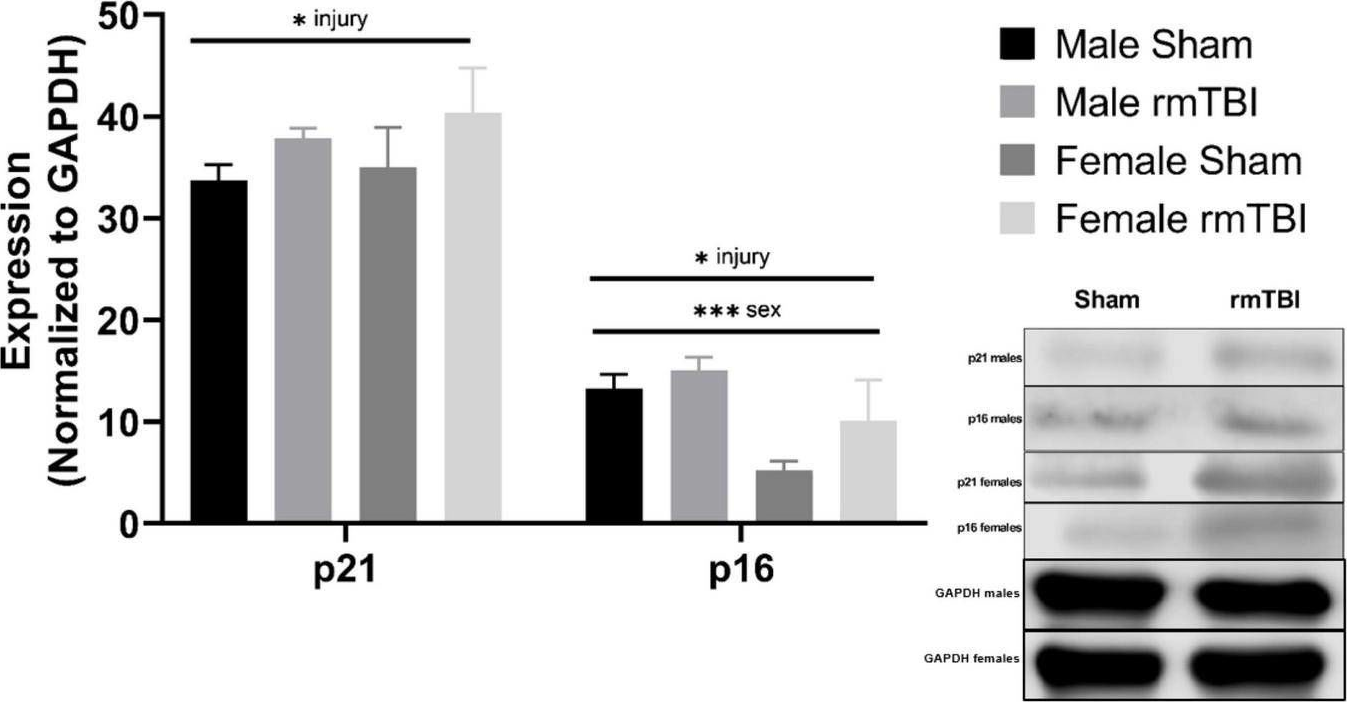
Fig.4.A
-
WB
-
Mus musculus (House mouse)
Collected and cropped from Frontiers in Neuroscience by CiteAb, provided under a CC-BY license
Image 1 of 70
In Commun Biol on 31 October 2022 by Ludikhuize, M. C., Gevers, S., et al.
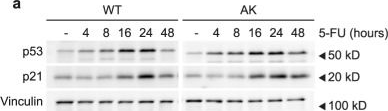
Fig.3.A
-
WB
-
Collected and cropped from Communications Biology by CiteAb, provided under a CC-BY license
Image 1 of 70
In Commun Biol on 31 October 2022 by Ludikhuize, M. C., Gevers, S., et al.
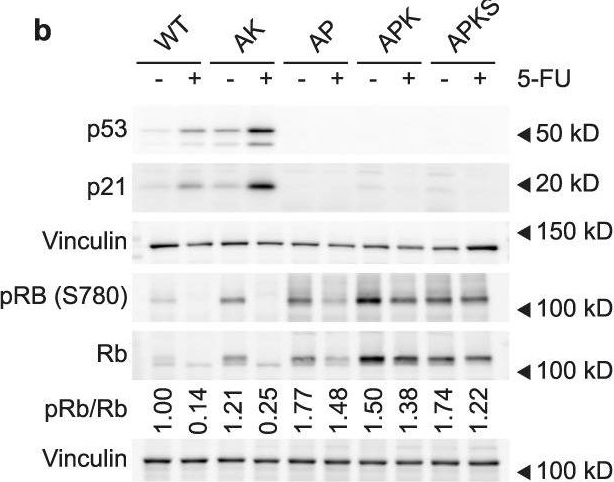
Fig.3.B
-
WB
-
Collected and cropped from Communications Biology by CiteAb, provided under a CC-BY license
Image 1 of 70
In Commun Biol on 31 October 2022 by Ludikhuize, M. C., Gevers, S., et al.
Fig.4.A
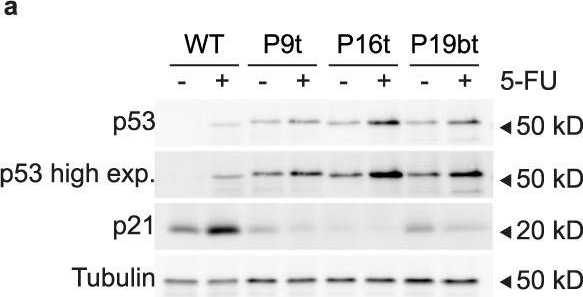
-
WB
-
Collected and cropped from Communications Biology by CiteAb, provided under a CC-BY license
Image 1 of 70
In Commun Biol on 31 October 2022 by Ludikhuize, M. C., Gevers, S., et al.
Fig.4.F
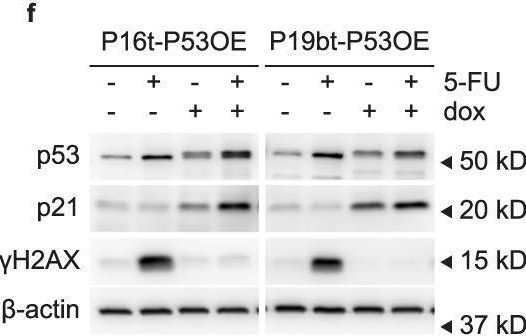
-
WB
-
Collected and cropped from Communications Biology by CiteAb, provided under a CC-BY license
Image 1 of 70
In Commun Biol on 31 October 2022 by Ludikhuize, M. C., Gevers, S., et al.
Fig.3.G
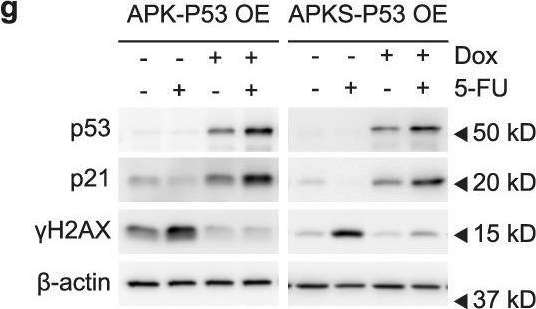
-
WB
-
Collected and cropped from Communications Biology by CiteAb, provided under a CC-BY license
Image 1 of 70
In BMC Mol Cell Biol on 14 July 2022 by Yuan, C. H., Hsu, W. C., et al.
Fig.5.A
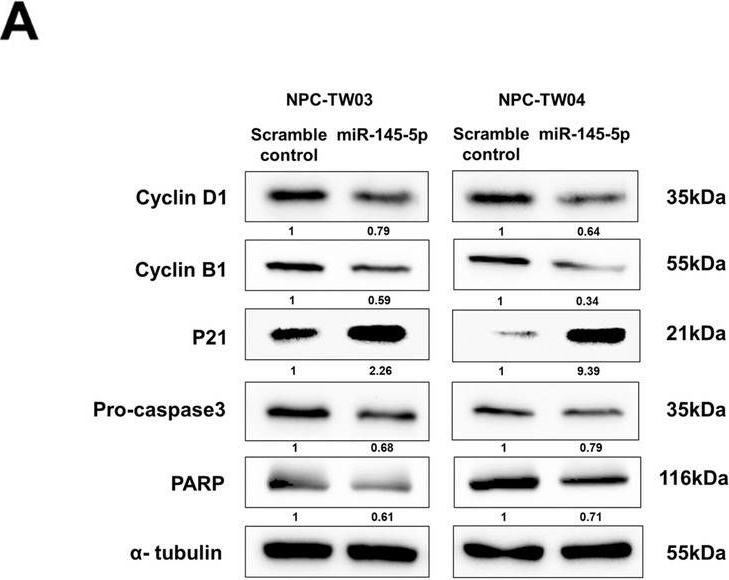
-
WB
-
Homo sapiens (Human)
Collected and cropped from BMC Molecular and Cell Biology by CiteAb, provided under a CC-BY license
Image 1 of 70
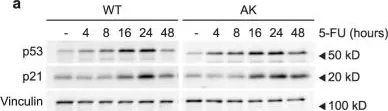
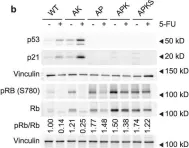
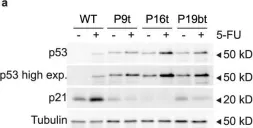
In Cell Death Dis on 28 June 2022 by Zois, C. E., Hendriks, A. M., et al.
Fig.3.C
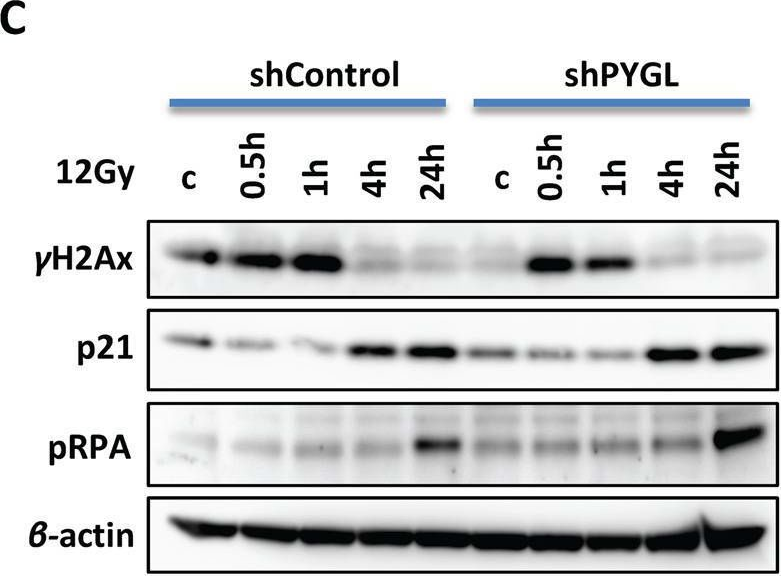
-
WB
-
Collected and cropped from Cell Death & Disease by CiteAb, provided under a CC-BY license
Image 1 of 70
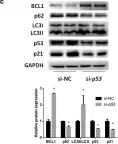
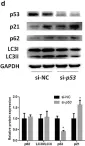
In Hepatol Int on 1 September 2020 by Zhang, X., Lin, Y., et al.
Fig.2.C
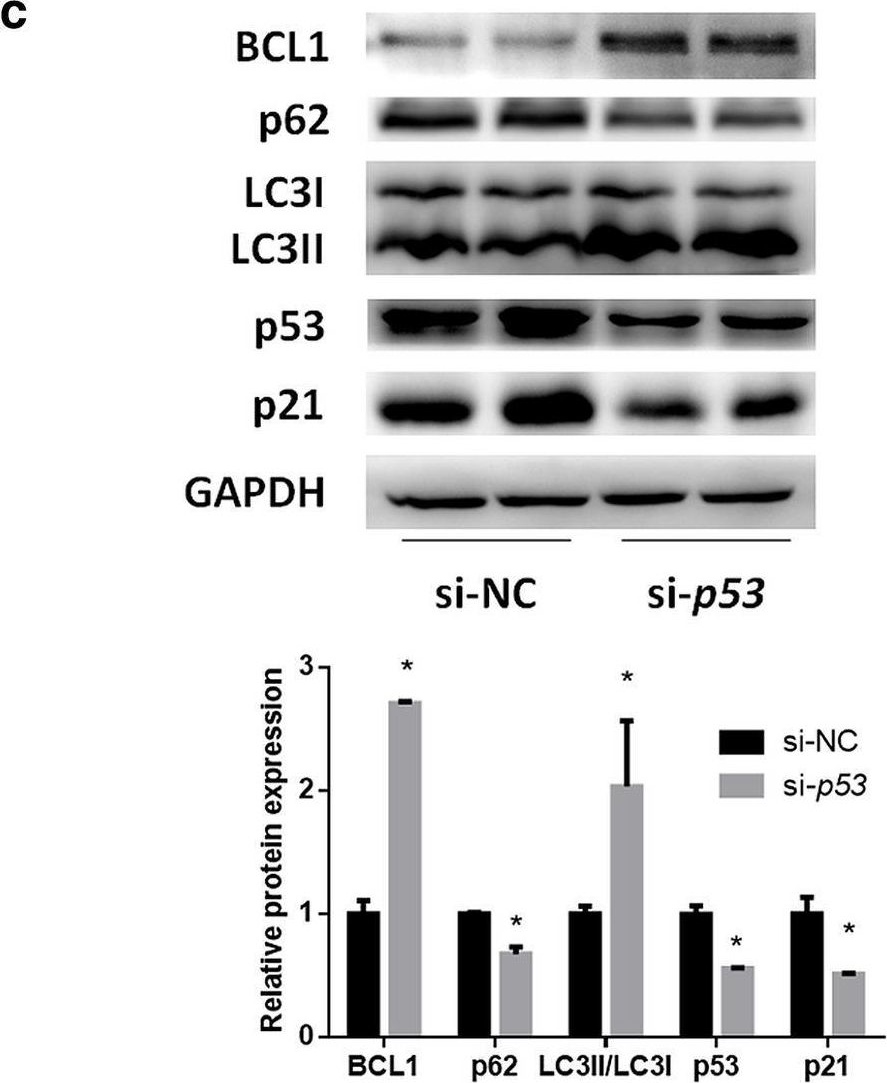
-
WB
-
Collected and cropped from Hepatology International by CiteAb, provided under a CC-BY license
Image 1 of 70
In Hepatol Int on 1 September 2020 by Zhang, X., Lin, Y., et al.
Fig.2.D
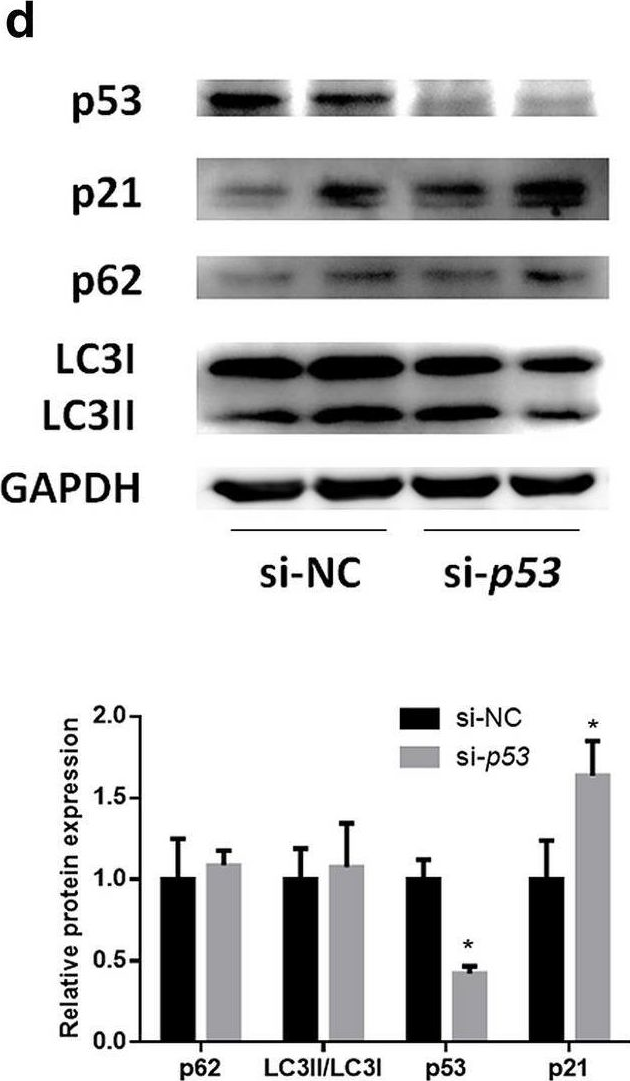
-
WB
-
Collected and cropped from Hepatology International by CiteAb, provided under a CC-BY license
Image 1 of 70
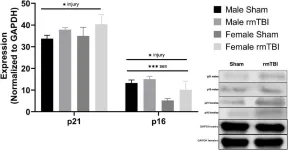
In Cell Death Dis on 27 July 2020 by Makarevich, O., Sabirzhanov, B., et al.
Fig.2.A
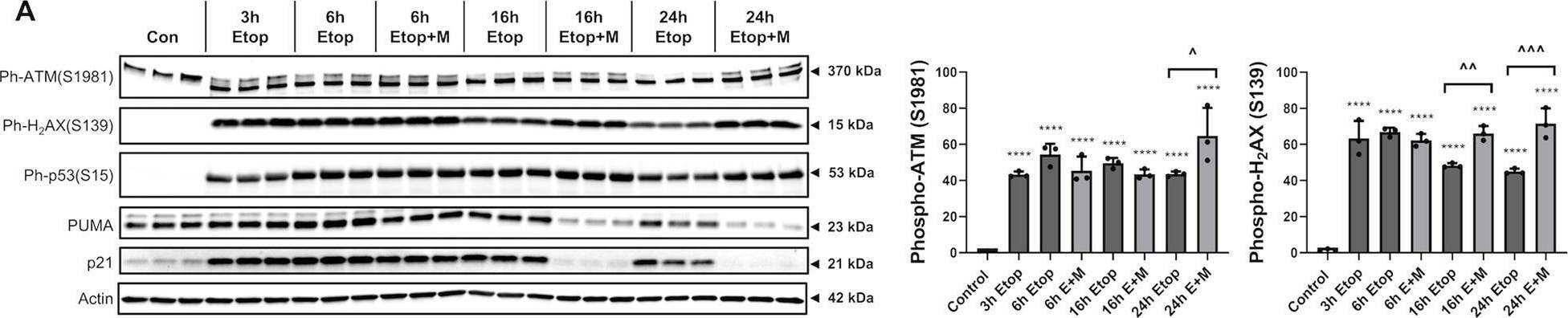
-
WB
-
Collected and cropped from Cell Death & Disease by CiteAb, provided under a CC-BY license
Image 1 of 70
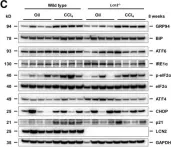
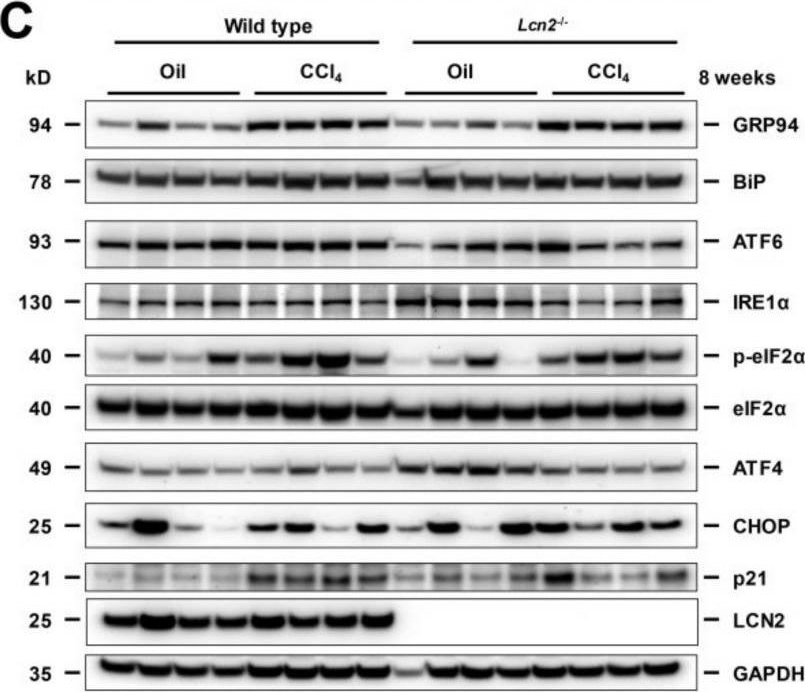
In Int J Mol Sci on 23 July 2020 by Borkham-Kamphorst, E., Haas, U., et al.
Fig.1.C
-
WB
-
Mus musculus (House mouse)
Collected and cropped from International Journal of Molecular Sciences by CiteAb, provided under a CC-BY license
Image 1 of 70
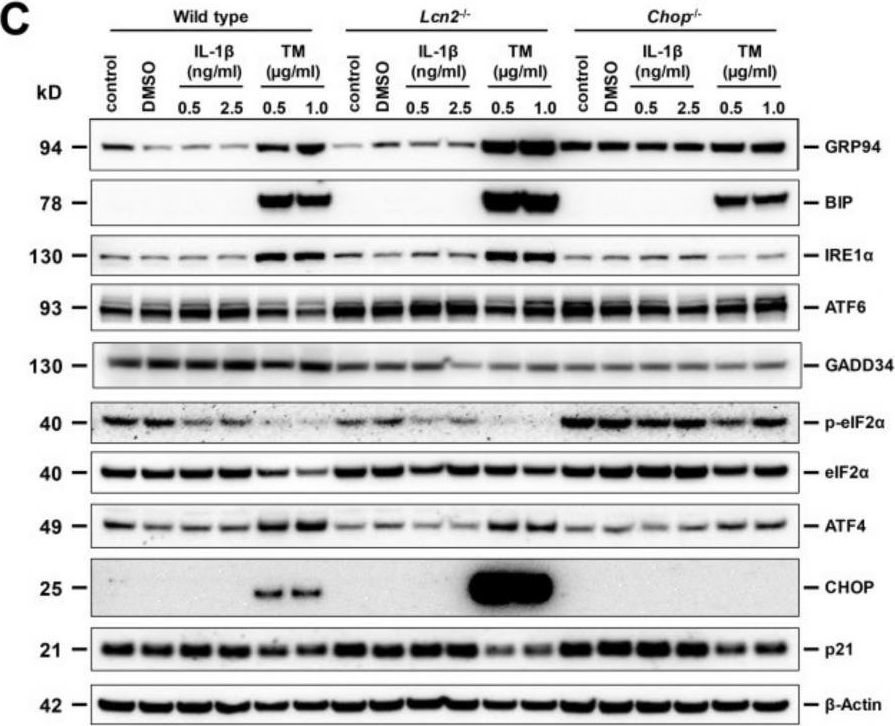
In Int J Mol Sci on 23 July 2020 by Borkham-Kamphorst, E., Haas, U., et al.
Fig.4.C
-
WB
-
Mus musculus (House mouse)
Collected and cropped from International Journal of Molecular Sciences by CiteAb, provided under a CC-BY license
Image 1 of 70