Pharmacological ascorbate (P-AscH, high-dose, intravenous, vitamin C), is a pro-drug that generates hydrogen peroxide (H2O2) and is being investigated as a neoadjuvant treatment for pancreatic adenocarcinoma (PDAC). In a randomized, phase II clinical trial, P-AscH demonstrated encouraging results in terms of efficacy and safety. However, some patients do not respond to P-AscH suggesting that resistance occurs in a subset of patients. The aims of this study were two-fold: first to characterize PDAC cells resistant to P-AscH, and second, determine if these alterations enhance metastatic potential. Resistance to P-AscH increased the ability to detoxify H2O2, altered redox metabolism and cell cycle regulation, however mechanisms to P-AscH resistance were different in the cell lines studied. Transcriptomic analysis demonstrated a significant enrichment of the epithelial-to-mesenchymal gene expression pattern in the cell lines studied, suggesting that upregulation of metastatic phenotypes occur during acquisition of resistance to P-AscH. Cells resistant to P-AscH demonstrated increased invasive potential, more aggressive tumor colonization, and higher abundance of circulating tumor cells in vivo. Our data support that resistance to oxidative stress enhances metastatic disease and indicates a potential route for PDAC to tolerate high levels of P-AscH and may explain why some patients do not respond to this treatment regimen.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Product Citations: 91
Pharmacologic ascorbate resistant pancreatic cancer demonstrates enhanced metastatic potential.
In Redox Biology on 1 July 2025 by Pope, A., O'Leary, B., et al.
-
Cancer Research
Preprint on BioRxiv : the Preprint Server for Biology on 23 December 2024 by Pedraza, N., Rocandio, D., et al.
During nervous system development, the interplay between cell cycle regulation and neurogenesis is fundamental to achieve the correct timing for neuronal differentiation. However, the molecular players regulating this transition are poorly understood. Among these, the cell-cycle regulatory cyclins and their cyclin-dependent kinases (Cdks) play a pivotal role. In the present work we uncover an unknown function of cyclin D1 (Ccnd1) during cortex development which is independent of cell cycle regulation and that relies on its cytoplasmic localization and membrane association. We show that Ccnd1 is localized in the cytoplasm of the radial glial process (RGP) of neuron progenitors in different regions of the developing brain, including the cortex. Cytoplasmic Ccnd1 is enriched at the distal tip of the RGP, adjacent to the meningeal basement membrane, and overlaps with β1-integrin at the plasma membrane. CCND1 knock-out animals show an abnormal cortical layering in which the distribution of Tbr2+ and Ctip2+ cells are affected without displaying proliferation defects. This is consistent with a cytoplasmic function of Ccnd1 as overexpression by in utero electroporation of a dominant negative Ccnd1, unable to activate Cdks, and targeted to the cytoplasmic membranes, reproduces some of these Tbr2 and Ctip2 defects. Finally, we provide evidence that cytoplasmic Ccnd1 affects neuron morphology and that it is required for the proper detachment of the RGP from the meningeal basement membrane by a mechanism involving the phosphorylation of the integrin effector protein paxillin. Hence, we propose that Ccnd1 has an important cytoplasmic function for cortical development independently of cell cycle regulation. Significant Statement A key developmental step during nervous system formation is the transition from proliferating progenitors to postmitotic neurons. However, the molecular mechanisms regulating this process are not fully understood. Cyclin D1 (Ccnd1) is a canonical regulator of cell cycle in the cell nucleus. Surprisingly, we show that Ccnd1 is also located in the radial glial process of neuron progenitors and associated to the plasma membrane in different regions of the developing mouse brain. We uncover a novel function for this cytoplasmic Ccnd1 and show that it is required for proper cortical layering, independent of cell cycle regulation. Mechanistically, we provide evidence that this function is mediated by the integrin effector paxillin. We propose therefore that cytoplasmic Ccnd1 is important for cortex development independent of cell cycle regulation.
-
WB
-
Cell Biology
-
Neuroscience
SOX10 mediates glioblastoma cell-state plasticity.
In EMBO Reports on 1 November 2024 by Man, K. H., Wu, Y., et al.
Phenotypic plasticity is a cause of glioblastoma therapy failure. We previously showed that suppressing the oligodendrocyte-lineage regulator SOX10 promotes glioblastoma progression. Here, we analyze SOX10-mediated phenotypic plasticity and exploit it for glioblastoma therapy design. We show that low SOX10 expression is linked to neural stem-cell (NSC)-like glioblastoma cell states and is a consequence of temozolomide treatment in animal and cell line models. Single-cell transcriptome profiling of Sox10-KD tumors indicates that Sox10 suppression is sufficient to induce tumor progression to an aggressive NSC/developmental-like phenotype, including a quiescent NSC-like cell population. The quiescent NSC state is induced by temozolomide and Sox10-KD and reduced by Notch pathway inhibition in cell line models. Combination treatment using Notch and HDAC/PI3K inhibitors extends the survival of mice carrying Sox10-KD tumors, validating our experimental therapy approach. In summary, SOX10 suppression mediates glioblastoma progression through NSC/developmental cell-state transition, including the induction of a targetable quiescent NSC state. This work provides a rationale for the design of tumor therapies based on single-cell phenotypic plasticity analysis.
© 2024. The Author(s).
In Radiation Research on 1 November 2023 by O'Leary, B. R., Kalen, A. L., et al.
Pharmacological ascorbate (P-AscH-, high dose, intravenous vitamin C) preferentially sensitizes human pancreas ductal adenocarcinoma (PDAC) cells to radiation-induced toxicity compared to non-tumorigenic epithelial cells. Radiation-induced G2-checkpoint activation contributes to the resistance of cancer cells to DNA damage induced toxicity. We hypothesized that P-AscH- induced radio-sensitization of PDAC cells is mediated by perturbations in the radiation induced activation of the G2-checkpoint pathway. Both non-tumorigenic pancreatic ductal epithelial and PDAC cells display decreased clonogenic survival and increased doubling times after radiation treatment. In contrast, the addition of P-AscH- to radiation increases clonogenic survival and decreases the doubling time of non-tumorigenic epithelial cells but decreasing clonogenic survival and increasing the doubling time of PDAC cells. Results from the mitotic index and propidium iodide assays showed that while the P-AscH- treatments did not affect radiation-induced G2-checkpoint activation, it enhanced G2-accumulation. The addition of catalase reverses the increases in G2-accumulation, indicating a peroxide-mediated mechanism. In addition, P-AscH- treatment of PDAC cells suppresses radiation-induced accumulation of cyclin B1 protein levels. Both translational and post-translational pathways appear to regulate cyclin B1 protein levels after the combination treatment of PDAC cells with P-AscH- and radiation. The protein changes seen are reversed by the addition of catalase suggesting that hydrogen peroxide mediates P-AscH- induced radiation sensitization of PDAC cells by enhancing G2-accumulation and reducing cyclin B1 protein levels.
©2023 by Radiation Research Society. All rights of reproduction in any form reserved.
-
WB
-
Cancer Research
Cap-dependent translation initiation monitored in living cells.
In Nature Communications on 2 November 2022 by Gandin, V., English, B. P., et al.
mRNA translation is tightly regulated to preserve cellular homeostasis. Despite extensive biochemical, genetic, and structural studies, a detailed understanding of mRNA translation regulation is lacking. Imaging methodologies able to resolve the binding dynamics of translation factors at single-cell and single-mRNA resolution were necessary to fully elucidate regulation of this paramount process. Here live-cell spectroscopy and single-particle tracking were combined to interrogate the binding dynamics of endogenous initiation factors to the 5'cap. The diffusion of initiation factors (IFs) changed markedly upon their association with mRNA. Quantifying their diffusion characteristics revealed the sequence of IFs assembly and disassembly in cell lines and the clustering of translation in neurons. This approach revealed translation regulation at high spatial and temporal resolution that can be applied to the formation of any endogenous complex that results in a measurable shift in diffusion.
© 2022. The Author(s).
-
WB
-
Mus musculus (House mouse)
-
Biochemistry and Molecular biology
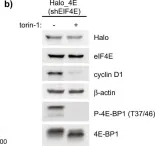
In Nat Commun on 2 November 2022 by Gandin, V., English, B. P., et al.
Fig.1.B

-
WB
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 4
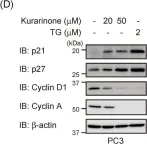
In Molecules on 27 August 2019 by Nishikawa, S., Itoh, Y., et al.
Fig.4.D

-
WB
-
Collected and cropped from Molecules by CiteAb, provided under a CC-BY license
Image 1 of 4
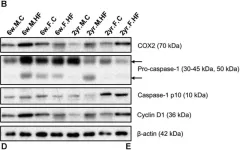
In Front Microbiol on 10 October 2018 by Lee, S. M., Kim, N., et al.
Fig.7.B

-
WB
-
Collected and cropped from Front Microbiol by CiteAb, provided under a CC-BY license
Image 1 of 4
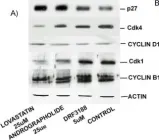
In BMC Cancer on 18 June 2004 by Satyanarayana, C., Deevi, D. S., et al.
Fig.3.A

-
WB
-
Collected and cropped from BMC Cancer by CiteAb, provided under a CC-BY license
Image 1 of 4