Aggregation has been widely described as a factor contributing to therapeutic antibody immunogenicity. Although production of high-affinity anti-drug antibodies depends on the activation of CD4 T lymphocytes, little is known about the T-cell response induced by antibody aggregates, especially for aggregates produced in mild conditions resulting from minor handling errors of vials. Large insoluble infliximab (IFX) aggregates produced in severe elevated temperature stress conditions have been previously shown to induce human monocyte-derived dendritic cell (moDC) maturation. We here showed that large IFX aggregates recruit in vitro a significantly higher number of CD4 T-cells compared to native IFX. Moreover, a larger array of T-cell epitopes encompassing the entire variable regions was evidenced compared to the native antibody. We then compared the responses of moDCs to different types of aggregates generated by submitting IFX to mild conditions of various times of incubation at an elevated temperature. Decreasing stress duration reduced aggregate size and quantity, and subsequently altered moDC activation. Of importance, IFX aggregates generated in mild conditions and not altering moDC phenotype generated an in vitro T-cell response with a higher frequency of CD4 T cells compared to native IFX. Moreover, cross-reactivity studies of aggregate-specific T cells showed that some T cells could recognize both native and aggregated IFX, while others responded only to IFX aggregates. Taken together, our results suggest that aggregation of antibodies in mild elevated temperature stress conditions is sufficient to alter moDC phenotype in a dose-dependent manner and to increase T-cell response.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Product Citations: 69
In European Journal of Pharmaceutical Sciences : Official Journal of the European Federation for Pharmaceutical Sciences on 1 January 2024 by Nabhan, M., Meunier, S., et al.
-
FC/FACS
-
Immunology and Microbiology
In Neoplasia (New York, N.Y.) on 1 November 2023 by Grottker, F., Gehre, S., et al.
Human papilloma virus (HPV) positive head and neck squamous cell carcinoma (HNSCC) tumors respond significantly better to anticancer treatments. It is assumed to be due to a better response to radiotherapy (RT), and presumably to an increased immunogenicity. However, little is known how the immune phenotype of HNSCC tumor cells is modulated by standard treatment, namely by radiochemotherapy (RCT).
Therefore, we aimed to examine the impact of the HPV status on the immune phenotype of HNSCC cell lines following RCT with 5 × 3Gy or 1 × 19.3Gy and/or docetaxel, by analyzing cell death, release of damage-associated molecular patterns (DAMPs), surface expression of immune checkpoint molecules (ICMs) and the impact on activation of human monocyte-derived dendritic cells (hmDCs).
Cell death induction and Hsp70 release following RCT was independent of the HPV status, and RCT significantly increased the expression of the immune suppressive ICMs PD-L1, PD-L2 and HVEM. An immune stimulatory ICM, CD137, was significantly increased following RCT only on HPV-positive cell lines, as well as the release of HMGB1. Although the treatment increased cell death and modulated ICM expression in HNSCC, the hmDCs were not activated after co-incubation with treated tumor cells.
Our data with the HPV-dependent release of HMGB1 and increased expression of CD137 following RCT provide a hint for increased immunogenicity underlining the better prognosis for HPV positive tumors following RCT.
Copyright © 2023. Published by Elsevier Inc.
-
FC/FACS
-
Homo sapiens (Human)
-
Cancer Research
-
Immunology and Microbiology
Tissue-like environments shape functional interactions of HIV-1 with immature dendritic cells.
In EMBO Reports on 5 June 2023 by Gallucci, L., Abele, T., et al.
Immature dendritic cells (iDCs) migrate in microenvironments with distinct cell and extracellular matrix densities in vivo and contribute to HIV-1 dissemination and mounting of antiviral immune responses. Here, we find that, compared to standard 2D suspension cultures, 3D collagen as tissue-like environment alters iDC properties and their response to HIV-1 infection. iDCs adopt an elongated morphology with increased deformability in 3D collagen at unaltered activation, differentiation, cytokine secretion, or responsiveness to LPS. While 3D collagen reduces HIV-1 particle uptake by iDCs, fusion efficiency is increased to elevate productive infection rates due to elevated cell surface exposure of the HIV-1-binding receptor DC-SIGN. In contrast, 3D collagen reduces HIV transfer to CD4 T cells from iDCs. iDC adaptations to 3D collagen include increased pro-inflammatory cytokine production and reduced antiviral gene expression in response to HIV-1 infection. Adhesion to a 2D collagen matrix is sufficient to increase iDC deformability, DC-SIGN exposure, and permissivity to HIV-1 infection. Thus, mechano-physical cues of 2D and 3D tissue-like collagen environments regulate iDC function and shape divergent roles during HIV-1 infection.
© 2023 The Authors. Published under the terms of the CC BY NC ND 4.0 license.
-
FC/FACS
-
Immunology and Microbiology
In The Journal of Immunology on 1 May 2023 by Alam, M. M., Gower, T., et al.
We have identified a combinational immunotherapy termed TheraVac vaccine (TheraVac) that can cure multiple large established mouse tumors, but it failed to cure melanoma in mice. TheraVac consists of an immunostimulating arm containing an agonist (HMGN1 [N1]) for TLR4 and an agonist (R848) for TLR7/8 that synergize to activate tumor-infiltrating dendritic cells (DCs) and promote Th1 immune responses. The second arm uses an immune checkpoint blockade, anti-PDL-1, to diminish tumor-associated immunosuppression. In this study, we investigated supplementation of TheraVac by a stimulator of IFN genes (STING) agonist, cyclic GMP-AMP (cGAMP), because together they synergize in activating DCs and produced more immunostimulating IL-12p70 and TNF-α cytokines. The synergistic activation and maturation of DCs is dependent on the activation of tank binding kinase-1 (TBK1). Treatment of three different melanin-producing mouse melanomas (B16F1, M3, and M4) with intratumoral delivery of cGAMP and TheraVac eradicated 60-80% of these melanomas. Immunoprofiling of M3 tumor treated with TheraVac plus cGAMP showed an increase in CD8+ CTLs and macrophages in the tumor. There was also a marked increase of CD4, CD8 effector and memory T cells and generation of functional tumor-specific CTLs in tumor-draining lymph nodes. The resultant tumor-free mice were selectively resistant to subsequent challenge with the same tumors, indicating long-term tumor-specific protective immunity. Overall, our findings have important implications for clinical trials with a combination of these immunotherapeutics to cure melanin-producing human melanomas, without the need for exogenous tumor Ags and no clear toxic effects in mice.
-
FC/FACS
-
Cancer Research
-
Immunology and Microbiology
In Cell Reports on 1 November 2022 by Deák, P., Studnitzer, B., et al.
Dendritic cell (DC) activation via pathogen-associated molecular patterns (PAMPs) is critical for antigen presentation and development of adaptive immune responses, but the stochastic distribution of DC responses to PAMP signaling, especially during the initial stages of immune activation, is poorly understood. In this study, we isolate a unique DC subpopulation via preferential phagocytosis of microparticles (MPs) and characterize this subpopulation of "first responders" (FRs). We present results that show these cells (1) can be isolated and studied via both increased accumulation of the micron-sized particles and combinations of cell surface markers, (2) show increased responses to PAMPs, (3) facilitate adaptive immune responses by providing the initial paracrine signaling, and (4) can be selectively targeted by vaccines to modulate both antibody and T cell responses in vivo. This study presents insights into a temporally controlled, distinctive cell population that influences downstream immune responses. Furthermore, it demonstrates potential for improving vaccine designs via FR targeting.Copyright © 2022. Published by Elsevier Inc.
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In PLoS One on 24 April 2008 by Schlaepfer, E. & Speck, R. F.
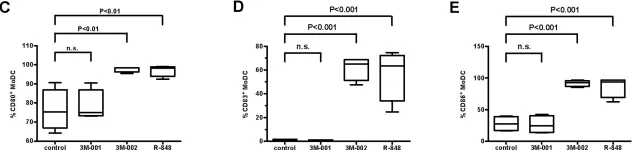
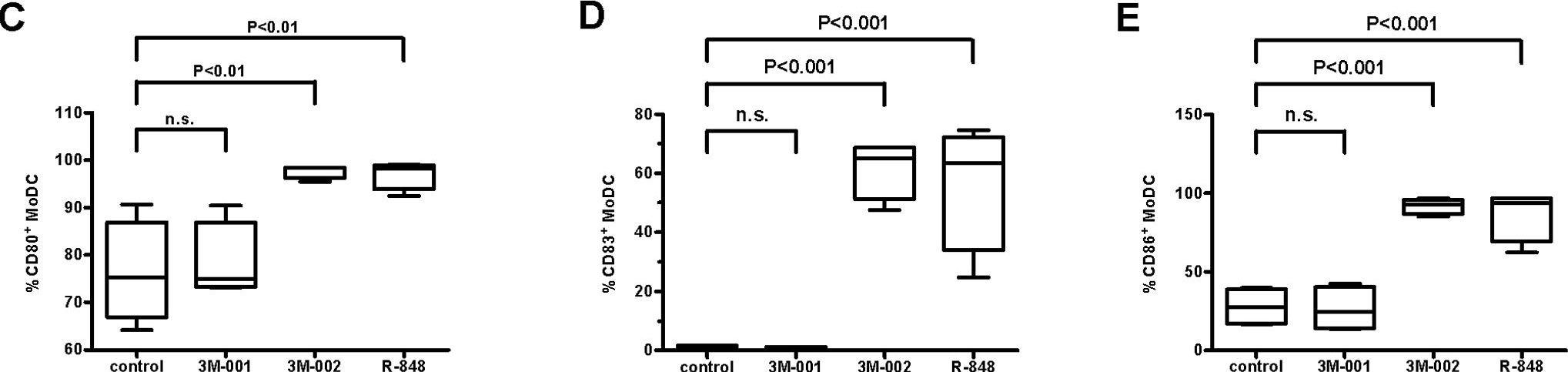
Fig.2.C,D,E
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from PLoS ONE by CiteAb, provided under a CC-BY license
Image 1 of 1