Gut microbes play a crucial role in regulating the tumor microenvironment (TME) of colorectal cancer (CRC). Nevertheless, the deep mechanism between the microbiota-TME interaction has not been well explored. In this study, we for the first time discovered that Lactobacillus intestinalis (L. intestinalis) effectively suppressed tumor growth both in the AOM/DSS-induced CRC model and the ApcMin/+ spontaneous adenoma model. Our investigation revealed that L. intestinalis increased the infiltration of immune cells, particularly dendritic cells (DC), in the TME. Mechanically, the tumor-derived CCL5 induced by L. intestinalis recruited DC chemotaxis through the NOD1/NF-κB signaling pathway. In clinical samples and datasets, we found positive correlation between L. intestinalis, CCL5 level, and the DC-related genes. Our study provided a new strategy for microbial intervention for CRC and deepened the understanding of the interaction between tumor cells and the immune microenvironment modulated by gut microbes.
Product Citations: 87
In Gut Microbes on 1 December 2025 by Sun, Y., Wang, Q., et al.
-
FC/FACS
-
Mus musculus (House mouse)
-
Cancer Research
-
Immunology and Microbiology
Fasting-mimicking diet remodels gut microbiota and suppresses colorectal cancer progression.
In NPJ Biofilms and Microbiomes on 25 June 2024 by Luo, M., Wang, Q., et al.
The progression of colorectal cancer is closely associated with diet. Fasting-mimicking diet (FMD) is a promising type of dietary intervention that have beneficial effects in the prevention and treatment of various cancers. We investigated the therapeutic effect of 4-day FMD against colorectal cancer in mice through immune cell analysis, microbiota composition analysis and anti-PD-1 treatment. These FMD cycles effectively suppressed colorectal cancer growth, reduced cell proliferation and angiogenesis, increased tumor-infiltration lymphocytes especially CD8+T cells. FMD stimulated protective gut microbiota, especially Lactobacillus. Supplementation of Lactobacillus johnsonii induced similar results as FMD intervention, which also suppressed tumor growth and increased CD45+ and CD8+ T cells. Additionally, FMD synthesizing with anti-PD-1 therapy effectively inhibited CRC progression. These findings suggest that Lactobacillus. johnsonii is necessary for the anticancer process of FMD in CRC. FMD through its effects on both gut microbiota and immune system, effectively suppressed colorectal cancer progression in mouse model.
© 2024. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Cancer Research
The disruptive effects of COPD exacerbation-associated factors on epithelial repair responses.
In Frontiers in Immunology on 24 June 2024 by Kortekaas, R. K., Geillinger-Kästle, K. E., et al.
Exacerbations of chronic obstructive pulmonary disease (COPD) increase mortality risk and can lead to accelerated loss of lung function. The increased inflammatory response during exacerbations contributes to worsening of airflow limitation, but whether it also impacts epithelial repair is unclear. Therefore, we studied the effect of the soluble factor micro-environment during COPD exacerbations on epithelial repair using an exacerbation cocktail (EC), composed of four factors that are increased in COPD lungs during exacerbations (IL-1β, IL-6, IL-8, TNF-α).
Mouse organoids (primary CD31-CD45-Epcam+ cells co-cultured with CCL206 fibroblasts) were used to study epithelial progenitor behavior. Mature epithelial cell responses were evaluated using mouse precision cut lung slices (PCLS). The expression of epithelial supportive factors was assessed in CCL206 fibroblasts and primary human lung fibroblasts.
EC exposure increased the number and size of organoids formed, and upregulated Lamp3, Muc5ac and Muc5b expression in day 14 organoids. In PCLS, EC imparted no effect on epithelial marker expression. Pre-treatment of CCL206 fibroblasts with EC was sufficient to increase organoid formation. Additionally, the expression of Il33, Tgfa and Areg was increased in CCL206 fibroblasts from EC treated organoids, but these factors individually did not affect organoid formation or size. However, TGF-α downregulated Foxj1 expression and upregulated Aqp5 expression in day 14 organoids.
EC exposure stimulates organoid formation and growth, but it alters epithelial differentiation. EC changes the epithelial progenitor support function of fibroblasts which contributes to observed effects on epithelial progenitors.
Copyright © 2024 Kortekaas, Geillinger-Kästle, Fuentes-Mateos, van Orsoy, Al-Alyan, Burgess and Gosens.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
Clec4A4 Acts as a Negative Immune Checkpoint Regulator to Suppress Antitumor Immunity.
In Cancer Immunology Research on 1 September 2023 by Uto, T., Fukaya, T., et al.
Clec4A4 is a C-type lectin receptor (CLR) exclusively expressed on murine conventional dendritic cells (cDC) to regulate their activation status. However, the functional role of murine Clec4A4 (mClec4A4) in antitumor immunity remains unclear. Here, we show that mClec4A4 serves as a negative immune checkpoint regulator to impair antitumor immune responses. Deficiency of mClec4A4 lead to a reduction in tumor development, accompanied by enhanced antitumor immune responses and amelioration of the immunosuppressive tumor microenvironment (TME) mediated through the enforced activation of cDCs in tumor-bearing mice. Furthermore, antagonistic mAb to human CLEC4A (hCLEC4A), which is the functional orthologue of mClec4A4, exerted protection against established tumors without any apparent signs of immune-related adverse events in hCLEC4A-transgenic mice. Thus, our findings highlight the critical role of mClec4A4 expressed on cDCs as a negative immune checkpoint molecule in the control of tumor progression and provide support for hCLEC4A as a potential target for immune checkpoint blockade in tumor immunotherapy.
©2023 The Authors; Published by the American Association for Cancer Research.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Journal of Experimental & Clinical Cancer Research : CR on 18 July 2023 by Lin, Y., Fan, L., et al.
The interplay between gut microbiota and tumor microenvironment (TME) in the pathogenesis of colorectal cancer (CRC) is largely unknown. Here, we elucidated the functional role of B. adolescentis and its possible mechanism on the manipulation of Decorin+ macrophages in colorectal cancer.
The relative abundance of B. adolescentis in tumor or para-tumor tissue of CRC patients was analyzed. The role of B. adolescentis was explored in the CRC animal models. The single cell-RNA sequencing (scRNA-seq) was used to investigate the myeloid cells subsets in TME. The expression level of TLR2/YAP axis and its downstream Decorin in macrophages were tested by Western blot and qRT-PCR. Knockdown of Decorin in Raw264.7 was performed to investigate the effect of Decorin+ macrophages on subcutaneous tumor formation. Multi-immunofluorescence assay examined the number of Decorin+ macrophages on the CRC tissue.
We found that the abundance of B. adolescentis was significantly reduced in tumor tissue of CRC patients. Supplementation with B. adolescentis suppressed AOM/DSS-induced tumorigenesis in mice. ScRNA-seq and animal experiment revealed that B. adolescentis increased Decorin+ macrophages. Mechanically, Decorin was activated by TLR2/YAP axis in macrophages. The abundance of B. adolescentis was correlated with the number of Decorin+ macrophages and the expression level of TLR2 in tumor tissue of CRC patients.
These results highlight that B. adolescentis induced Decorin+ macrophages and provide a novel therapeutic target for probiotic-based modulation of immune microenvironment in CRC.
© 2023. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Cancer Research
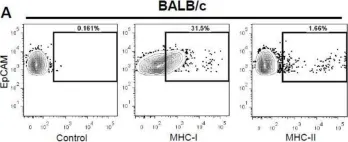
In Int J Mol Sci on 6 April 2018 by Bockerstett, K. A., Wong, C. F., et al.
Fig.3.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 3
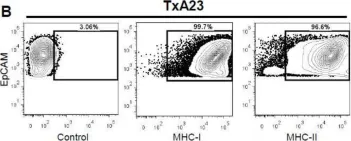
In Int J Mol Sci on 6 April 2018 by Bockerstett, K. A., Wong, C. F., et al.
Fig.3.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 3
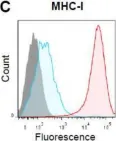
In Int J Mol Sci on 6 April 2018 by Bockerstett, K. A., Wong, C. F., et al.
Fig.3.C

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 3