Elevated hexosamine biosynthesis fuels tumor growth by facilitating protein and lipid glycosylation. But which enzyme in this pathway is better to serve as an antitumor target remains unclear. Here, we revealed that targeting GFAT1, the rate-limiting enzyme in hexosamine synthesis, exhibits limited inhibitory effects on glioblastoma (GBM), the most lethal brain tumor. This outcome is due to the compensation of NAGK-mediated hexosamine salvage pathway. Unexpectedly, inhibiting PGM3, which controls the flux of both de novo hexosamine synthesis and salvage pathways, down-regulates the expression of other enzymes in this pathway and suppresses SREBP-1, a critical lipogenic transcription factor, effectively inhibiting GBM growth. Unexpectedly, SREBP-1 transcriptionally up-regulates the expression of hexosamine synthesis enzymes, while inhibition of these enzymes in turn down-regulates SREBP-1 activation via reducing N-glycosylation of its transporter, SCAP. Our study identified PGM3 as a promising target for treating GBM. Its inhibition disrupts the SREBP-1 activation-hexosamine synthesis positive feedback regulation to effectively eliminate GBM cells.
Product Citations: 53
In Science Advances on 18 April 2025 by Su, H., Zhong, Y., et al.
-
Cancer Research
Comprehensive understanding of context-specific functions of PHF2 in lipid metabolic tissues.
In Scientific Reports on 17 March 2025 by Jeong, D. W., Yun, J. E., et al.
Adipose tissue and the liver are known to regulate lipid metabolism through the storage, synthesis, and breakdown of lipids. However, the shared molecular factors affecting lipid metabolism in both tissues remain unclear. Plant Homeodomain Finger 2 (PHF2), one of the histone lysine demethylase7 family, serves as an epigenetic regulator in adipose tissue and E3 ubiquitin ligase in liver cancer. This study uses bioinformatics to analyze the role of PHF2 in lipid metabolism, focusing on its functions in adipose tissue and the liver. We utilized cDNA-chip microarrays, public clinical data, and in vitro three-dimensional cell culture models, validating our bioinformatics findings. Consequently, our analyses showed that PHF2 is positively involved in histone demethylase activity and adipogenesis in patients with obesity and moderate liver disease. However, PHF2 suppressed de novo lipogenesis and tumor progression in patients with liver cancer, enhancing immune cell infiltration in liver cancer. Furthermore, it was experimentally confirmed that PHF2 increases lipid accumulation in adipocytes but acts as a tumor suppressor in liver cancer cells. Overall, this study provides a comprehensive overview of PHF2, highlighting its biological importance.
© 2025. The Author(s).
-
WB
-
Homo sapiens (Human)
-
Biochemistry and Molecular biology
-
Cell Biology
Preprint on Research Square on 5 February 2025 by Wang, Y., Wei, Y., et al.
Abstract Background Colorectal carcinoma (CRC) is the third most prevalent cancer worldwide and a leading cause of cancer-related mortality, primarily due to its high propensity for metastasis. Despite therapeutic advancements, the molecular mechanisms by which intratumor hypoxia and oxidative stress contribute to malignancy remain poorly understood. Methods Gene expression profiles were analyzed across various stages of CRC (T1–T4) and metastatic CRC (mCRC). The role of hypoxia-inducible factor 1 alpha (HIF1α) was investigated in CRC cell lines and in vivo models to evaluate its impact on local tumor growth and lung metastasis. Mechanistic studies focused on HIF1α-mediated regulation of fatty acid synthase (FASN) and GLUT3 in the tumor microenvironment to promote CRC distant metastasis. Therapeutic targeting of HIF1α was assessed using echinomycin encapsulated in lipid nanoparticles. Results HIF1α was identified as a critical regulator enabling tumors to overcome intratumoral stress and proliferate in hypoxic regions. Activation of the IGF1/Insulin-AKT-mTOR pathway led to HIF1α accumulation, which, in turn, upregulated FASN and GLUT3 expression, mitigating oxidative and hypoxic stress. Elevated HIF1α levels were positively correlated with increased NRF2 activity in CRC cells, contributing to malignancy and metastatic potential. Targeting the HIF1α-FASN/GLUT3 axis with echinomycin-loaded lipid nanoparticles effectively inhibited tumor growth and abrogated lung metastases in preclinical models. Conclusions The IGF1/Insulin-HIF1α-FASN/GLUT3 signaling axis is a key driver of CRC progression and lung metastasis by counteracting oxidative stress and hypoxia. Therapeutic inhibition of HIF1α offers promising potential for mitigating CRC malignancy and preventing metastasis.
-
Cancer Research
In Cell Reports Medicine on 17 September 2024 by Zhong, Y., Geng, F., et al.
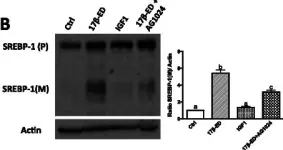
Antipsychotic drugs have been shown to have antitumor effects but have had limited potency in the clinic. Here, we unveil that pimozide inhibits lysosome hydrolytic function to suppress fatty acid and cholesterol release in glioblastoma (GBM), the most lethal brain tumor. Unexpectedly, GBM develops resistance to pimozide by boosting glutamine consumption and lipogenesis. These elevations are driven by SREBP-1, which we find upregulates the expression of ASCT2, a key glutamine transporter. Glutamine, in turn, intensifies SREBP-1 activation through the release of ammonia, creating a feedforward loop that amplifies both glutamine metabolism and lipid synthesis, leading to drug resistance. Disrupting this loop via pharmacological targeting of ASCT2 or glutaminase, in combination with pimozide, induces remarkable mitochondrial damage and oxidative stress, leading to GBM cell death in vitro and in vivo. Our findings underscore the promising therapeutic potential of effectively targeting GBM by combining glutamine metabolism inhibition with lysosome suppression.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
-
Biochemistry and Molecular biology
-
Cell Biology
In Experimental and Therapeutic Medicine on 1 August 2024 by Liu, C., Pan, X., et al.
Hyperlipidemia is a strong risk factor for numerous diseases. Resveratrol (Res) is a non-flavonoid polyphenol organic compound with multiple biological functions. However, the specific molecular mechanism and its role in hepatic lipid metabolism remain unclear. Therefore, the aim of the present study was to elucidate the mechanism underlying how Res improves hepatic lipid metabolism by decreasing microRNA-33 (miR-33) levels. First, blood miR-33 expression in participants with hyperlipidemia was detected by reverse transcription-quantitative PCR, and the results revealed significant upregulation of miR-33 expression in hyperlipidemia. Additionally, after transfection of HepG2 cells with miR-33 mimics or inhibitor, western blot analysis indicated downregulation and upregulation, respectively, of the mRNA and protein expression levels of sirtuin 6 (SIRT6). Luciferase reporter analysis provided further evidence for binding of miR-33 with the SIRT6 3'-untranslated region. Furthermore, the levels of peroxisome proliferator-activated receptor-γ (PPARγ), PPARγ-coactivator 1α and carnitine palmitoyl transferase 1 were increased, while the concentration levels of acetyl-CoA carboxylase, fatty acid synthase and sterol regulatory element-binding protein 1 were decreased when SIRT6 was overexpressed. Notably, Res improved the basic metabolic parameters of mice fed a high-fat diet by regulating the miR-33/SIRT6 signaling pathway. Thus, it was demonstrated that the dysregulation of miR-33 could lead to lipid metabolism disorders, while Res improved lipid metabolism by regulating the expression of miR-33 and its target gene, SIRT6. Thus, Res can be used to prevent or treat hyperlipidemia and associated diseases clinically by suppressing hepatic fatty acid synthesis and increasing fatty acid β-oxidation.
Copyright: © 2024 Liu et al.
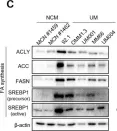
In Cancers (Basel) on 30 June 2023 by Han, A., Mukha, D., et al.
Fig.1.C

-
WB
-
Collected and cropped from Cancers (Basel) by CiteAb, provided under a CC-BY license
Image 1 of 9
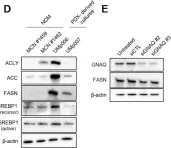
In Cancers (Basel) on 30 June 2023 by Han, A., Mukha, D., et al.
Fig.1.D

-
WB
-
Collected and cropped from Cancers (Basel) by CiteAb, provided under a CC-BY license
Image 1 of 9
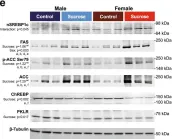
In Nat Commun on 13 October 2022 by Stephenson, E. J., Stayton, A. S., et al.
Fig.2.E

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 9
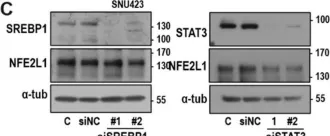
In Cancers (Basel) on 15 September 2020 by Lee, Y. K., Kwon, S. M., et al.
Fig.3.C

-
WB
-
Collected and cropped from Cancers (Basel) by CiteAb, provided under a CC-BY license
Image 1 of 9
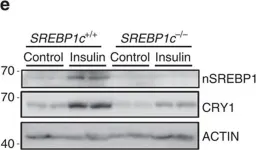
In Nat Commun on 14 July 2016 by Jang, H., Lee, G. Y., et al.
Fig.2.B

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 9
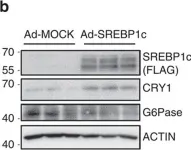
In Nat Commun on 14 July 2016 by Jang, H., Lee, G. Y., et al.
Fig.1.B

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 9
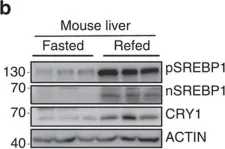
In Nat Commun on 14 July 2016 by Jang, H., Lee, G. Y., et al.
Fig.2.E

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 9
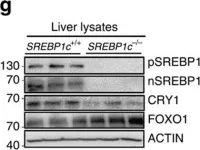
In Nat Commun on 14 July 2016 by Jang, H., Lee, G. Y., et al.
Fig.4.G

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 9
In BMC Cancer on 29 May 2015 by Belkaid, A., Duguay, S. R., et al.
Fig.7.B

-
WB
-
Collected and cropped from BMC Cancer by CiteAb, provided under a CC-BY license
Image 1 of 9