Antipsychotic drugs have been shown to have antitumor effects but have had limited potency in the clinic. Here, we unveil that pimozide inhibits lysosome hydrolytic function to suppress fatty acid and cholesterol release in glioblastoma (GBM), the most lethal brain tumor. Unexpectedly, GBM develops resistance to pimozide by boosting glutamine consumption and lipogenesis. These elevations are driven by SREBP-1, which we find upregulates the expression of ASCT2, a key glutamine transporter. Glutamine, in turn, intensifies SREBP-1 activation through the release of ammonia, creating a feedforward loop that amplifies both glutamine metabolism and lipid synthesis, leading to drug resistance. Disrupting this loop via pharmacological targeting of ASCT2 or glutaminase, in combination with pimozide, induces remarkable mitochondrial damage and oxidative stress, leading to GBM cell death in vitro and in vivo. Our findings underscore the promising therapeutic potential of effectively targeting GBM by combining glutamine metabolism inhibition with lysosome suppression.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Product Citations: 56
In Cell Reports Medicine on 17 September 2024 by Zhong, Y., Geng, F., et al.
-
Biochemistry and Molecular biology
-
Cell Biology
In American Journal of Cancer Research on 10 May 2024 by Liu, J. P., Shen, K. Y., et al.
Chronic inflammation associated with lung cancers contributes to immunosuppressive tumor microenvironments, reducing CD8+ T-cell function and leading to poor patient outcomes. A disintegrin and metalloprotease domain 9 (ADAM9) promotes cancer progression. Here, we aim to elucidate the role of ADAM9 in the immunosuppressive tumor microenvironment. A bioinformatic analysis of TIMER2.0 was used to investigate the correlation of ADAM9 and to infiltrate immune cells in the human lung cancer database and mouse lung tumor samples. Flow cytometry, immunohistochemistry, and RNA sequencing (RNA-seq) were performed to investigate the ADAM9-mediated immunosuppressive microenvironment. The coculture system of lung cancer cells with immune cells, cytokine array assays, and proteomic approach was used to investigate the mechanism. By analyzing the human LUAD database and the mouse lung cancer models, we showed that ADAM9 was associated with the immunosuppressive microenvironment. Additionally, ADAM9 released IL6 protein from cancer cells to inhibit IL12p40 secretion from dendritic cells, therefore leading to dendritic cell dysfunction and further affecting T-cell functions. Proteomic analysis indicated that ADAM9 promoted cholesterol biosynthesis and increased IL6-STAT3 signaling. Mechanistically, ADAM9 reduced the protein stability of LDLR, resulting in reduced cholesterol uptake and induced cholesterol biosynthesis. Moreover, LDLR reduction enhanced IL6-STAT3 activation. We reveal that ADAM9 has a novel biological function that drives the immunosuppressive tumor microenvironment by linking lung cancer's metabolic and signaling axes. Thus, by targeting ADAM9 an innovative and promising therapeutic opportunity was indicated for regulating the immunosuppression of lung cancer.
AJCR Copyright © 2024.
-
WB
-
Cancer Research
In IScience on 15 March 2024 by Singh, S., Wright, R. E., et al.
Zika virus (ZIKV) infection during pregnancy causes severe neurological and ocular abnormalities in infants, yet no vaccine or antivirals are available. Our transcriptomic analysis of ZIKV-infected retinal pigment epithelial (RPE) cells revealed alterations in the cholesterol pathway. Thus, we investigated the functional roles of ATP binding cassette transporter G1 (ABCG1) and sterol response element binding protein 2 (SREPB-2), two key players in cholesterol metabolism, during ocular ZIKV infection. Our in vitro data showed that increased ABCG1 activity via liver X receptors (LXRs), reduced ZIKV replication, while ABCG1 knockdown increased replication with elevated intracellular cholesterol. Conversely, inhibiting SREBP-2 or its knockdown reduced ZIKV replication by lowering cholesterol levels. In vivo, LXR agonist or SREBP-2 inhibitor treatment mitigated ZIKV-induced chorioretinal lesions in mice, concomitant with decreased expression of inflammatory mediators and increased activation of antiviral response genes. In summary, our study identifies ABCG1's antiviral role and SREBP-2's proviral effects in ocular ZIKV infection, offering cholesterol metabolism as a potential target to develop antiviral therapies.
© 2024 The Author(s).
-
Immunology and Microbiology
-
Pathology
In American Journal of Cancer Research on 11 October 2023 by Lin, J. C., Liu, T. P., et al.
Effective therapies for hepatocellular carcinoma (HCC) are urgently needed, as it is a type of cancer resistant to chemotherapy. Recent evidence showed that PF-429242, a membrane-bound transcription factor site-1 protease (MBTPS1) inhibitor, exhibited anticancer activities against glioblastomas, renal cell carcinoma, and pancreatic cancer. However, its anticancer activity against HCC has yet to be investigated. In this study, we found that PF-429242 induced autophagy-dependent cell death in HCC cells. RNA-sequencing analysis indicated that the primary effect of PF-429242 was inhibition of the sterol regulatory element-binding protein (SREBP) signaling pathway. However, overexpression of SREBP proteins did not efficiently rescue PF-429242-induced autophagy and cell death. Mechanistically, PF-429242 induced forkhead box protein O1 (FOXO1)-dependent autophagic cell death. Additionally, PF-429242 caused FOXO1-independent upregulation of insulin-like growth factor-binding protein 1 (IGFBP1), ultimately leading to autophagy-independent cell death. The in vivo anticancer activity of PF-429242 against HCC cells was demonstrated in a tumor xenograft mouse model. Therefore, PF-429242 is a potential anticancer agent to treat HCC by triggering FOXO1-dependent autophagic cell death and IGFBP1-mediated anti-survival signaling in parallel.
AJCR Copyright © 2023.
-
WB
-
Cancer Research
In Journal of Food and Drug Analysis on 31 August 2023 by Chen, W. H., Guo, B. C., et al.
Statins induce nitric oxide (NO) bioavailability by activating endothelial nitric oxide synthase via kinase- and calcium-dependent pathways in endothelial cells (ECs). However, their effect on the metabolism of L-arginine, the precursor for NO biosynthesis, and regulatory mechanism have not yet been investigated. In this study, we investigated the role of the autophagy-urea cycle-L-arginine pathway in simvastatin-mediated NO bioavailability in ECs. Griess's assay was used to determine the NO bioavailability. Protein expression was assessed using Western blot analysis. Further, immunocytochemistry was performed to observe autophagosome formation, while conventional assay kits were used to quantify the levels of different intermediate substrates of the urea cycle. In ECs, treatment with simvastatin induced the activation of autophagy flux, as evidenced by the increased levels of microtubule-associated protein 1A/1B-light chain 3 II and autophagolysosome formation and decreased levels of p62. Inhibition of autophagy by ATG7 small interfering RNA (siRNA), chloroquine and bafilomycin A1 abolished simvastatin-induced NO bioavailability, EC proliferation, migration, and tube formation. Additionally, simvastatin increased the intermediate substrates levels of the urea cycle, including glutamate, acetyl-CoA, urea, and L-arginine, all of which were abrogated by chloroquine or bafilomycin A1. Genetic knockdown of argininosuccinate lyase using siRNA abrogated simvastatin-induced increase in NO bioavailability and EC-related functions. Moreover, inhibition of AMP-activated protein kinase (AMPK) and transient receptor potential vanilloid 1 (TRPV1) prevented simvastatin-induced activation of the autophagy-urea cycle pathway and NO production. Our findings suggest that simvastatin activates the autophagy-urea cycle pathway via TRPV1-AMPK signaling, which increases L-arginine bioavailability and ultimately promotes NO production in ECs.
-
Homo sapiens (Human)
-
Cell Biology
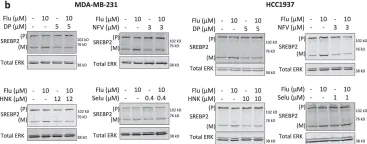
In Nat Commun on 24 October 2022 by van Leeuwen, J. E., Ba-Alawi, W., et al.
Fig.3.B

-
WB
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 6
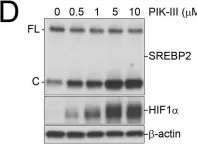
In PLoS One on 25 August 2020 by Kobylarz, M. J., Goodwin, J. M., et al.
Fig.3.D

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 6
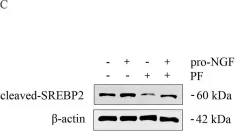
In Cell Death Dis on 11 July 2019 by Pham, D. D., Bruelle, C., et al.
Fig.2.C

-
WB
-
Homo sapiens (Human)
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 6
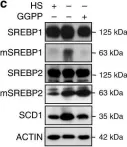
In Nat Commun on 22 March 2019 by Bertolio, R., Napoletano, F., et al.
Fig.1.C

-
WB
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 6
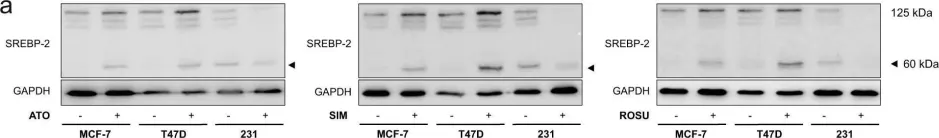
In Cell Death Dis on 28 January 2019 by Göbel, A., Breining, D., et al.
Fig.4.A

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 6
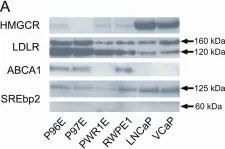
In PLoS One on 5 July 2012 by Murtola, T. J., Syvälä, H., et al.
Fig.3.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 6