Adaptive immunity relies on dendritic cell (DC) migration to transport antigens from tissues to lymph nodes. Galectins, a family of β-galactoside-binding proteins, control cell membrane organization, exerting crucial roles in multiple physiological processes. Here, we report a novel mechanism underlying cell polarity and uropod retraction by demonstrating that galectin-9 regulates basal and chemokine-driven DC migration in humans and mice. Galectin-9 depletion caused a defect in RhoA signaling that resulted in impaired cell rear contractility. Mechanistically, galectin-9 interacts with and organizes CD44 at the cell surface, in turn modulating RhoA binding to GEF-H1 and the initiation of downstream signaling. Analysis of DC motility in the 3D tumor microenvironment revealed galectin-9 is also required for DC recruitment and infiltration. Exogenous galectin-9 rescued the motility of tumor-immunocompromised human blood DCs, validating the physiological relevance of galectin-9 in DC migration. Our results identify galectin-9 as a necessary mechanistic component for DC motility by regulating cell polarity and contractility, and underscore its implications for DC-based immunotherapies.
© 2025 Franken et al.
Product Citations: 113
Galectin-9 regulates dendritic cell polarity and uropod contraction by modulating RhoA activity.
In The Journal of Cell Biology on 3 November 2025 by Franken, G. A., Warner, H., et al.
-
FC/FACS
-
Cell Biology
-
Immunology and Microbiology
Differential response of human plasmacytoid pre-dendritic cells to SARS-CoV-2 variants.
In IScience on 19 September 2025 by Kartasheva-Ebertz, D., Topalis, D., et al.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants have been involved in various waves of the COVID-19 pandemic and showed different pathogenicity and inflammatory potential. Whether they can induce different patterns of innate immune activation in antigen-presenting cells is poorly understood. Here, we investigated the ability of primary plasmacytoid pre-dendritic cells (pDC), type 2 dendritic cells (DC2), and monocytes isolated from healthy donors to respond to SARS-CoV-2 variants. Transcriptomic profiling using RNA sequencing revealed that pDC respond differentially to SARS-CoV-2 variants, unlike DC2 and monocytes. Functional studies showed that pDC undergo differential activation programs upon SARS-CoV-2 variant stimulation. The Alpha and Delta variants induced P1-/P2-pDC effector phenotypes, characterized by strong IFN-α production. In contrast, the Omicron variant predominantly triggered a T cell-activating P3 phenotype, with lower IFN-α and IFN-λ production, and stronger proinflammatory and CD4+T cell responses. Our results indicate that SARS-CoV-2 variants can control pDC diversification pattern in different ways, which may influence disease severity.
© 2025 INSERM. Published by Elsevier Inc.
-
FC/FACS
-
COVID-19
-
Immunology and Microbiology
In Nature Communications on 25 August 2025 by Sequera, C., Grattarola, M., et al.
Histone deacetylases (HDACs) are epigenetic regulators frequently altered in cancer. Here we report that overexpression of HDAC1/2 occurs in Hepatocellular Carcinoma (HCC) patients, correlating with poor prognosis. We show that romidepsin, a class-I HDAC inhibitor, elicits a combinatorial perturbation of distinct molecular processes in HCC cells, altering lipid composition, mitotic spindle machinery, and levels of cell cycle/survival signals. Collectively, these alterations lead HCC cells to a vulnerable state, conferring dependency to receptor tyrosine kinase (RTK) signalling support. The cytostatic effects of romidepsin alone is converted into cytotoxicity by the RTK inhibitor cabozantinib in HCC models. We document that romidepsin+cabozantibib confers an immune-stimulatory profile in Alb-R26Met mouse models, with direct effects on primary human dendritic cell maturation in vitro. Our findings put forward the intricate crosstalk between epigenetics, metabolism, and immune response in cancer. The broad action of romidepsin on distinct cellular functions highlights its therapeutic potential for HCC treatment.
© 2025. The Author(s).
-
Cancer Research
Activated T Cells Break Tumor Immunosuppression by Macrophage Reeducation.
In Cancer Discovery on 3 July 2025 by Trotta, R., Rivis, S., et al.
In this study, we observe that in human and murine melanomas, T-cell activation abates hematopoietic prostaglandin-D2 synthase (HPGDS) transcription in tumor-associated macrophages (TAM) through TNFα signaling. Mechanistically, HPGDS installs a prostaglandin D2 (PGD2) autocrine loop in TAMs via DP1 and DP2 activation that sustains their protumoral phenotype and promotes paracrine inhibition of CD8+ T cells via a PGD2-DP1 axis. Genetic or pharmacologic HPGDS targeting induces antitumoral features in TAMs and favors CD8+ T-cell recruitment, activation, and cytotoxicity, altogether sensitizing tumors to αPD1. Conversely, HPGDS overexpression in TAMs or systemic TNFα blockade sustains a protumoral environment and αPD1 resistance, preventing the downregulation of HPGDS by T cells. Congruently, patients and mice resistant to αPD1 fail to suppress HPGDS in TAMs, reinforcing the evidence that circumventing HPGDS is necessary for efficient αPD1 treatment. Overall, we disclose a mechanism whereby T-cell activation controls the innate immune system, and we suggest HPGDS/PGD2 targeting to overcome immunotherapy resistance.
In this study, we show a mechanism whereby T-cell activation controls the innate immune system and shapes the tumor microenvironment by reducing PGD2 production in TAMs. We suggest HPGDS inhibition as a promising strategy to treat refractory tumors to current immunotherapies or to overcome acquired resistance to immune checkpoint blockade.
©2025 The Authors; Published by the American Association for Cancer Research.
-
FC/FACS
-
Cancer Research
-
Immunology and Microbiology
In Frontiers in Immunology on 3 June 2025 by Chen, Y., Wang, J., et al.
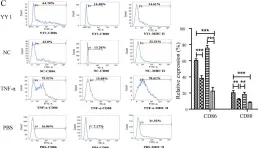
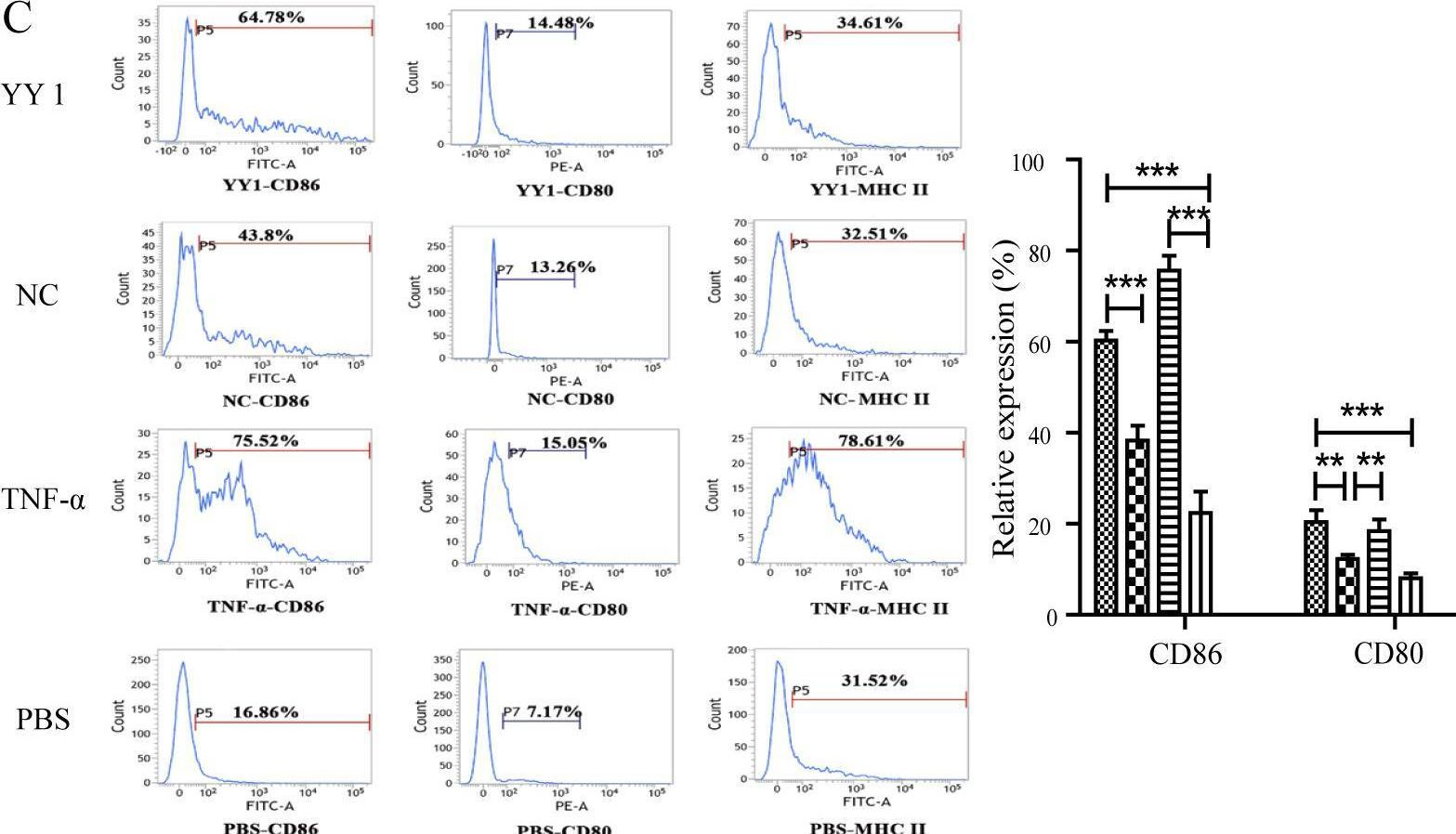
Acute rejection is a critical complication after liver transplantation, contributing significantly to transplant dysfunction and recipient mortality. Yin Yang 1 (YY1), a zinc-finger transcription factor, has an undefined role in liver allograft acute rejection, despite its broad expression and regulatory potential in immune responses.
To investigate YY1's role, we used an MHC Class II-mismatched rat liver transplantation model. Allografts were harvested on post-transplant days 5 and 10 for YY1 expression analysis in inflammatory cells around recipient liver central veins. In vitro, dendritic cells (DCs) were transfected to overexpress YY1, and their surface markers (CD80, CD86, MHC II) and cytokine production (TNF-α, IL-6) were assessed. Naïve CD4+ T cells were co-cultured with YY1-overexpressing DCs to evaluate their polarization towards inflammatory phenotypes (IL-17, IFN-γ production).
YY1 expression was elevated in inflammatory cells of allografts on days 5 and 10 post-transplant, correlating with increased serum transaminases and inflammatory cytokines. YY1-overexpressing DCs showed heightened expression of CD80, CD86, and MHC II, along with augmented TNF-α and IL-6 production. These YY1-activated DCs drove naïve CD4+ T cells to produce higher levels of IL-17 and IFN-γ, indicating polarization towards a proinflammatory Th17/Th1 phenotype.
YY1 promotes DC activation and naïve T cell polarization towards inflammatory phenotypes, thereby contributing to acute rejection in liver transplantation. Targeting YY1 may offer a therapeutic strategy to mitigate acute rejection and improve transplant outcomes. Further research is warranted to explore YY1's regulatory mechanisms and therapeutic potential in liver transplantation.
Copyright © 2025 Chen, Wang, Hong, Wang, He and Chen.
-
FC/FACS
-
Immunology and Microbiology
In Front Immunol on 3 June 2025 by Chen, Y., Wang, J., et al.
Fig.4.C
-
FC/FACS
-
Collected and cropped from Frontiers in Immunology by CiteAb, provided under a CC-BY license
Image 1 of 4
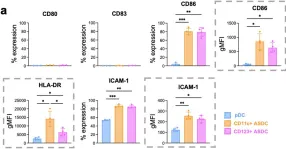
In PLoS Pathog on 1 June 2024 by Warner van Dijk, F. A., Tong, O., et al.
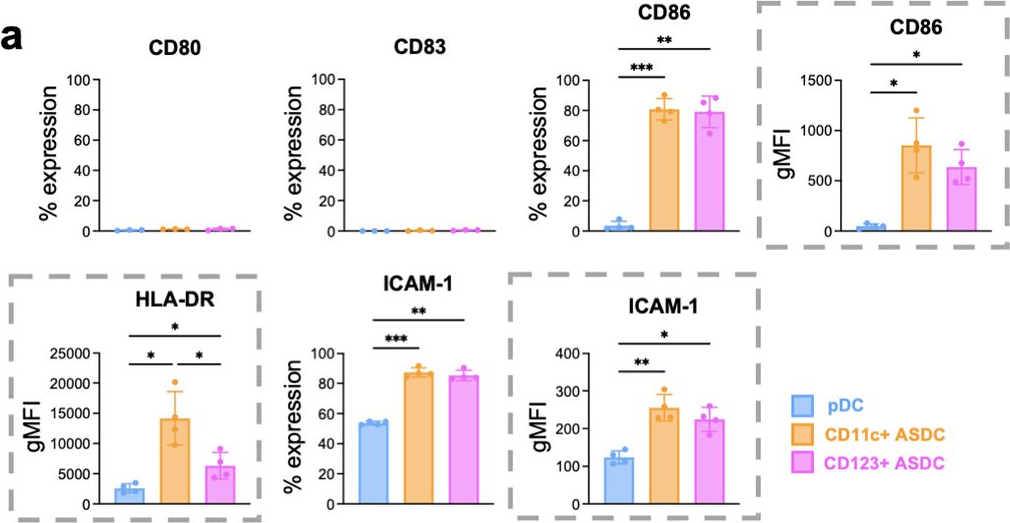
Fig.6.A
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from PLoS Pathogens by CiteAb, provided under a CC-BY license
Image 1 of 4
In PLoS Pathog on 1 November 2009 by Cavaleiro, R., Baptista, A. P., et al.
Fig.3.B
-
FC/FACS
-
Collected and cropped from PLoS Pathogens by CiteAb, provided under a CC-BY license
Image 1 of 4
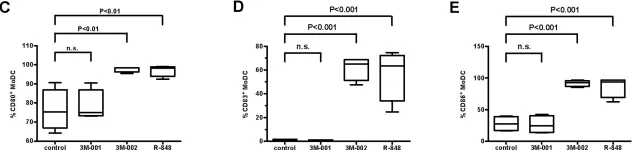
In PLoS One on 24 April 2008 by Schlaepfer, E. & Speck, R. F.
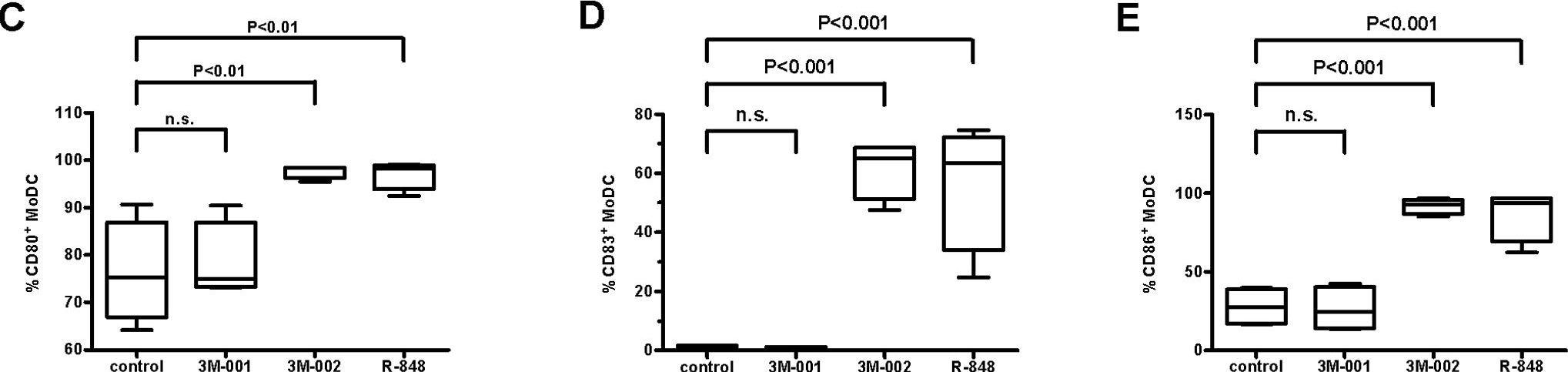
Fig.2.C,D,E
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from PLoS ONE by CiteAb, provided under a CC-BY license
Image 1 of 4