Ischemic stroke, one of the leading causes of death in the world, is accompanied by the dysfunction of the blood-brain barrier (BBB), which aggravates neuron damage. However, the mechanisms underlying the restoration of BBB in the chronic stage after stroke remain unclear. Here, pericyte pool alterations and their consequences for BBB integrity and brain recovery were analyzed in the C57BL/6 mice stroke model. Lineage tracing, RNA-seq, and immunofluorescence staining revealed endothelial cell (EC) transdifferentiation into pericytes (E-pericytes) in C57BL/6 mice after stroke. E-pericytes depletion by diphtheria toxin A (DTA) aggravated BBB leakage and exacerbated neurological deficits in the MCAO model. The myeloid cell-driven transdifferentiation of ECs into pericytes accelerated BBB restoration and brain self-repair after stroke via endothelial-mesenchymal transformation (EndoMT). Decreasing the number of E-pericytes by specific knockout of the Tgfbr2 gene in ECs also aggravated BBB leakage and exacerbated neurological deficits. EC-specific overexpression of the Tgfbr2 gene promoting E-pericytes transdifferentiation reduced BBB leakage and exerted neuroprotective effects. Deciphering the mechanism by which E-pericytes coordinate post-stroke recovery may reveal a novel therapeutic opportunity.
© 2025, Li et al.
Product Citations: 249
In eLife on 16 July 2025 by Li, T., Yang, L., et al.
-
Cardiovascular biology
In Breast Cancer Research : BCR on 23 June 2025 by Corrado, A., Lorito, N., et al.
Metabolic alterations, including acidosis in the tumor microenvironment, have been extensively linked to more aggressive phenotypes and increased therapy resistance. However, current imaging techniques are limited in their ability to capture extracellular tumor acidosis precisely and assess spatial heterogeneity in vivo, making its association with augmented malignancy poorly understood. In this study, we investigated whether Magnetic Resonance Imaging- Chemical Exchange Saturation Transfer (MRI-CEST) technique for tumor pH imaging of intratumoral acidosis could differentiate between metastatic and non-metastatic breast cancers.
Isogenic metastatic (4T1) and non-metastatic (67NR) breast cancer cell lines were characterized for their metabolic and acidosis features, including LDH-A/PDK-1 expression, glucose consumption, extracellular acidification rate (ECAR) and oxygen consumption rate (OCR). Potential relationship between tumor acidosis, vascularization and hypoxia with metastatic potential was assessed in vivo by MRI-based imaging approaches in orthotopic breast tumors. Validation of MRI findings was assessed ex vivo by western blot, immunohistochemistry and immunofluorescence assays for a multiparametric characterization of tumor microenvironment and metabolic properties.
We observed a higher energetic profile of the 4T1 cells compared to the 67NR cells, alongside elevated glycolytic (LDH-A, PDK-1), hypoxia (CAIX, Pimonidazole), and vascularization (CD31) markers in 4T1 orthotopic primary tumors, which were associated with a greater metastatic propensity. MRI-CEST tumor pH imaging revealed increased extracellular tumor acidity in 4T1 tumors, along with marked spatial intratumoral heterogeneity, in contrast to the more homogenous 67NR tumors, as further confirmed by LAMP-2 staining. Notably, this spatial intratumor heterogeneity in acidosis enables clear differentiation between high- and low-malignancy tumors.
These findings underscore the role of tumor acidosis and its spatial heterogeneity in promoting aggressive phenotypes and highlight the potential of in vivo tumor pH imaging as a marker of malignancy in breast cancers.
© 2025. The Author(s).
-
Cancer Research
In Theranostics on 11 April 2025 by Du, M., Zhao, X., et al.
Background: Diabetic retinopathy (DR) is a vision-threatening microvascular complication of diabetes mellitus. Chronic inflammation and endothelial dysfunction are critical factors in the disease's pathogenesis. Consequently, interventions developed to reduce retinal inflammation are anticipated to be beneficial for both the prevention and treatment of DR. In the present study, we developed a unique class of drugless peptide-based nanohybrids with potent anti-inflammatory activities and investigated their therapeutic efficacy for treating DR in an oxygen-induced retinopathy (OIR) mouse model and a streptozotocin (STZ)-induced diabetic mouse model. Methods: Hexapeptides were applied to modify gold nanoparticles to form the drugless peptide-based nanohybrids (P12). We then examined the physicochemical properties and anti-inflammatory activities of P12 in HUVECs and BV2 cells and identified the critical amino acids for this novel bioactivity. The intravitreal and retro-orbital injections were applied to determine the optimal retinal delivery route for P12. The therapeutic efficacy of P12 in treating DR were investigated using both the OIR model and STZ-induced diabetic model. Through immunohistochemistry and flow cytometry analyses, we identified the major cells that internalize P12 in the retina. Furthermore, in vitro experiments were used to explore the underlying molecular mechanisms for the anti-inflammatory activities of P12. Results: We found that P12 exhibited potent anti-inflammatory effects in both HUVECs and BV2 cells. In addition, P12 can be efficiently delivered to the retina via intravitreal injection. Intravitreally injected P12 significantly improved early DR symptoms including vascular leakage and pericyte loss in STZ-induced diabetic mice. It also suppressed pathological neovascularization and retinal hemorrhage in OIR mice. Importantly, we found that intravitreally injected P12 was mainly taken up by microglial and endothelial cells, leading to reduced retinal endothelium inflammation and microglial activation in DR animal models. Mechanistic studies revealed that P12 potently inhibited several TLR4 downstream signaling pathways, such as NF-κB, JNK, and P38 MAPK, in both endothelial and microglial cells. This effect is due to the capacity of P12 in blocking the endosomal acidification process that governs the endosomal TLR signaling transduction. Conclusions: Our findings suggest that local injection of properly designed, drugless, peptide-based nanohybrids can serve as a safe and effective anti-inflammatory nanomedicine for treating DR.
© The author(s).
-
Immunology and Microbiology
-
Neuroscience
Preprint on Research Square on 1 April 2025 by Xia, P., Lee, S., et al.
Abstract Cardiac complications, including myocardial injury and dysfunction, are common in severe viral respiratory infections (VRI) and are associated with increased mortality1–3. The pathophysiology of VRI-induced myocardial injury is multifactorial, but frequently involves structural damage to the heart’s microvascular network that leads to subsequent myocardial ischemia and dysfunction4–6. Currently, there are no targeted therapies available to prevent or attenuate VRI-associated myocardial injury. Moreover, the molecular mechanisms driving the cardiac microvascular pathology in severe VRI are largely unclear. In this study, we identify increased endothelial cell (EC) activin type IIA receptor (ActRIIA) signaling as a key mediator of cardiac microvascular injury and pathologic remodeling in severe VRI. We show that genetic deletion of EC ActRIIA is sufficient to mitigate EC death and myocardial capillary loss in a murine model of severe influenza infection, which results in improved myocardial perfusion, cardiac function, and survival. We then provide proof-of-concept evidence for two novel pharmacological approaches to target EC ActRIIA pathophysiology in the treatment of VRI-induced cardiac dysfunction.
-
Cardiovascular biology
-
Immunology and Microbiology
In Disease Models & Mechanisms on 1 April 2025 by Jafree, D. J., Perera, C., et al.
Therapies targeting blood vessels hold promise for autosomal dominant polycystic kidney disease (ADPKD), the most common inherited disorder causing kidney failure. However, the onset and nature of kidney vascular abnormalities in ADPKD are poorly defined. Accordingly, we employed a combination of single-cell transcriptomics; three-dimensional imaging with geometric, topological and fractal analyses; and multimodal magnetic resonance imaging with arterial spin labelling to investigate aberrant microvasculature in ADPKD kidneys. Within human ADPKD kidneys with advanced cystic pathology and excretory failure, we identified a molecularly distinct blood microvascular subpopulation, characterised by impaired angiogenic signalling and metabolic dysfunction, differing from endothelial injury profiles observed in non-cystic human kidney diseases. Next, Pkd1 mutant mouse kidneys were examined postnatally, when cystic pathology is well established, but before excretory failure. An aberrant endothelial subpopulation was also detected, concurrent with reduced cortical blood perfusion. Disorganised kidney cortical microvasculature was also present in Pkd1 mutant mouse fetal kidneys when tubular dilation begins. Thus, aberrant features of cystic kidney vasculature are harmonised between human and mouse ADPKD, supporting early targeting of the vasculature as a strategy to ameliorate ADPKD progression.
© 2025. Published by The Company of Biologists.
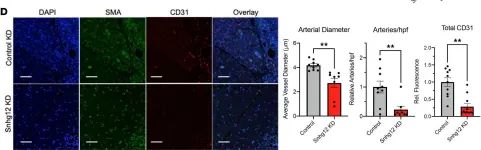
In JCI Insight on 11 January 2022 by Gross, D. A., Cheng, H. S., et al.
Fig.6.D

-
IHC-IF
-
Mus musculus (House mouse)
Collected and cropped from JCI Insight by CiteAb, provided under a CC-BY license
Image 1 of 6
In Sci Rep on 4 February 2019 by Murakami, Y., Naganuma, H., et al.
Fig.3.L

-
IHC-IF
-
Mus musculus (House mouse)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 6
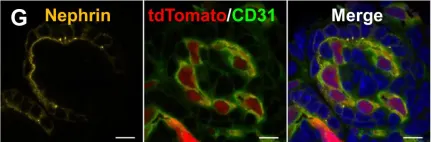
In Sci Rep on 4 February 2019 by Murakami, Y., Naganuma, H., et al.
Fig.5.G

-
IHC-IF
-
Mus musculus (House mouse)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 6
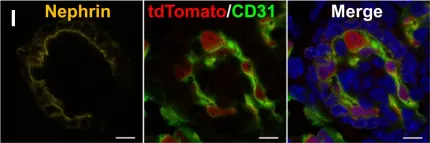
In Sci Rep on 4 February 2019 by Murakami, Y., Naganuma, H., et al.
Fig.5.I

-
IHC-IF
-
Mus musculus (House mouse)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 6
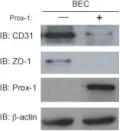
In BMC Dev Biol on 28 June 2010 by Kim, H., Nguyen, V. P., et al.
Fig.5.B

-
WB
-
Mus musculus (House mouse)
Collected and cropped from BMC Dev Biol by CiteAb, provided under a CC-BY license
Image 1 of 6
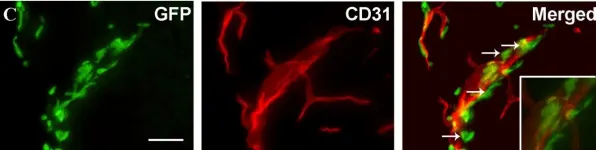
In J Neuroinflammation on 23 October 2008 by Clausen, B. H., Lambertsen, K. L., et al.
Fig.3.C

-
IHC-IF
-
Mus musculus (House mouse)
Collected and cropped from J Neuroinflammation by CiteAb, provided under a CC-BY license
Image 1 of 6