Cellular senescence is deeply involved in physiological homeostasis, development, tissue repair, aging, and diseases. Senescent cells (SnCs) accumulate in aged tissues and exert deleterious effects by secreting proinflammatory molecules that contribute to chronic inflammation and aging-related diseases. We revealed that an aberrant interaction between glycolytic PGAM1 and Chk1 kinase is augmented in SnCs associated with increased glycolysis, whose byproduct, lactate, promotes this binding in a noncell autonomous manner. The pseudo-Warburg effect of SnCs with enhanced PPP (pentose phosphate pathway) activity is maintained by HIF-2α phosphorylation by Chk1 and subsequent upregulation of glycolytic enzymes, creating a vicious cycle reprogramming the glycolytic pathway in SnCs. HIF-2α also activates FoxM1 expression, which transcriptionally suppresses proapoptotic profiles, including BIM, and upregulates DNA repair machineries in SnCs. FoxM1 thus supports the genomic integrity and survival capacity of SnCs during their glycolytic changes. Chemical abrogation of PGAM1-Chk1 binding reverts these phenotypes and eliminates SnCs through senolysis. Inhibition of the PGAM1-Chk1 interaction improves physiological parameters during aging and inhibits lung fibrosis in mouse models. Our study highlights a novel pathway contributing to the metabolic reprogramming of SnCs and how the use of a new senolytic molecule that targets the PGAM-Chk1 interaction creates a specific vulnerability of those cells to potentially fight age-related diseases.
© 2025. The Author(s).
Product Citations: 41
In Signal Transduction and Targeted Therapy on 15 December 2025 by Mikawa, T., Kameda, M., et al.
In EMBO Molecular Medicine on 1 April 2025 by Lu, Z., Stencel, O., et al.
Viral infections pose a significant global burden. Host susceptibility to pathogens is determined by many factors including genetic variation that can lead to immunodeficient or dysregulated antiviral immune responses. Pax5 heterozygosity (Pax5-/+), resulting in reduced PAX5 levels in mice, mimics germline or somatic PAX5 dysregulation contributing to diseases such as childhood B-cell precursor acute lymphoblastic leukemia (B-ALL). In contrast to the well-characterized roles of PAX5 during early B-cell development, little is known about how Pax5 heterozygosity impacts antiviral responses. We infected Pax5-/+ mice with the noncytopathic Lymphocytic Choriomeningitis Virus (LCMV) and found that infection with the chronic Docile strain resulted in decreased survival of Pax5-/+ mice. While early adaptive CD8+ T-cell (CTL) immunity was robust in Pax5-/+ mice, LCMV-specific neutralizing antibody production was compromised leading to impaired long-term viral clearance and a pro-inflammatory milieu in the bone marrow (BM). Here we show that survival outcomes were improved upon prophylactic treatment with the β-glucan immune trainer through induction of heterologous protection against chronic infection. β-Glucan enhanced viral clearance, CTL immunity, neutralizing antibody production and reduced monocyte immunosuppression in multiple LCMV-resident host organs. New insight from this study will help design effective prophylactic treatment strategies against chronic viral infections, particularly in genetically predisposed susceptible hosts.
© 2025. The Author(s).
-
Biochemistry and Molecular biology
-
Immunology and Microbiology
In Cellular and Molecular Life Sciences : CMLS on 26 March 2025 by Montilla, A., Zabala, A., et al.
Interferon regulatory factor 5 (IRF5) is a transcription factor that plays a role in orchestrating innate immune responses, particularly in response to viral infections. Notably, IRF5 has been identified as a microglia risk gene linked to multiple sclerosis (MS), but its specific role in MS pathogenesis remains unclear. Through the use of Irf5-/- mice, our study uncovers a non-canonical function of IRF5 in MS recovery. Irf5-/- mice exhibited increased damage in an experimental autoimmune encephalomyelitis (EAE) model and demonstrated impaired oligodendrocyte recruitment into the lesion core following lysolecithin-induced demyelination. Transcriptomic and lipidomic analyses revealed that IRF5 has a role in microglia-mediated myelin phagocytosis, lipid metabolism, and cholesterol homeostasis. Indeed, Irf5-/- microglia phagocytose myelin, but myelin debris is not adequately degraded, leading to an accumulation of lipid droplets, cholesterol esters, and cholesterol crystals within demyelinating lesions. This abnormal buildup can hinder remyelination processes. Importantly, treatments that promote cholesterol transport were found to reduce lipid droplet accumulation and mitigate the exacerbated damage in Irf5-/- mice with EAE. Altogether, our study identified the antiviral transcription factor IRF5 as a key transcriptional regulator of lipid degradation and cholesterol homeostasis and suggest that loss of IRF5 function leads to pathogenic lipid accumulation in microglia, thereby obstructing remyelination. These data and the fact that Irf5 polymorphisms are significantly associated with MS, highlight IRF5 as a potential therapeutic target to promote regenerative responses.
© 2025. The Author(s).
-
Biochemistry and Molecular biology
-
Cell Biology
-
Neuroscience
In PLoS ONE on 27 February 2025 by Toriu, N., Sato, Y., et al.
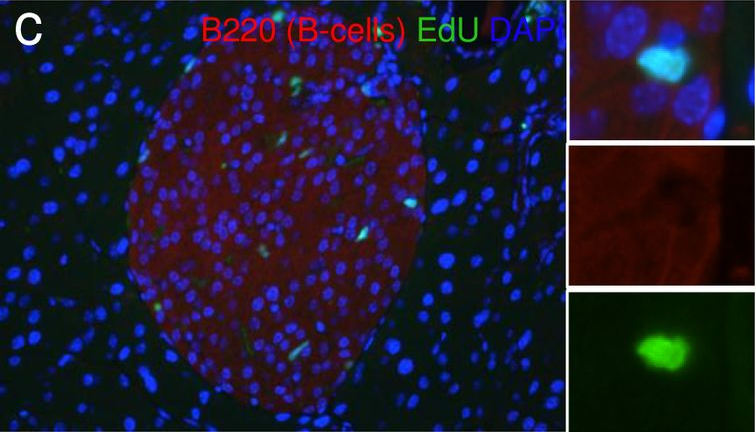
Tertiary lymphoid tissues (TLTs) are ectopic lymphoid structures induced by multiple stimuli, including infection and tissue injuries; however, their clinical relevance in disease progression has remained unclear. We demonstrated previously that TLTs develop in mouse and human kidneys with aging and can be a potential marker of kidney injury and prognosis, and therapeutic targets. In addition, we found that two types of unique lymphocytes that emerge with aging, senescence-associated T cells and age-associated B cells, are essential for TLT formation in the kidney. Although TLTs develop with aging in other organs as well, their cellular and molecular components, and clinical significance remain unclear. In the present study, we found that TLTs developed in the liver with aging, and that their cellular and molecular components were similar to those in the kidneys. Notably, senescence-associated T cells and age-associated B cells were also present in hepatic TLTs. Furthermore, analysis of publicly available data on human liver biopsy transcriptomes revealed that the expression of TLT-related genes was elevated in the liver biopsy samples from hepatitis C virus (HCV)-infected patients compared with those without HCV infection and was associated with liver injury and fibrosis. Therefore, we analyzed liver biopsy samples from 47 HCV patients and found that TLTs were present in 87.2% of cases and that the numbers and stages of TLTs were higher in aged patients and cellular and molecular components of TLTs in humans were similar to those in mice. Our findings suggesting that age-dependent TLT formation is a systemic phenomenon across the tissues and aging is also a predisposing factor for TLT formation across organs.
Copyright: © 2025 Toriu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
-
IHC-IF
-
Mus musculus (House mouse)
In Cell Death and Differentiation on 1 June 2024 by Bosso, G., Cintra Herpst, A. C., et al.
The BRAF gene is mutated in a plethora of human cancers. The majority of such molecular lesions result in the expression of a constitutively active BRAF variant (BRAFV600E) which continuously bolsters cell proliferation. Although we recently addressed the early effects triggered by BRAFV600E-activation, the specific contribution of ERK1 and ERK2 in BRAFV600E-driven responses in vivo has never been explored. Here we describe the first murine model suitable for genetically dissecting the ERK1/ERK2 impact in multiple phenotypes induced by ubiquitous BRAFV600E-expression. We unveil that ERK1 is dispensable for BRAFV600E-dependent lifespan shortening and for BRAFV600E-driven tumor growth. We show that BRAFV600E-expression provokes an ERK1-independent lymphocyte depletion which does not rely on p21CIP1-induced cell cycle arrest and is unresponsive to ERK-chemical inhibition. Moreover, we also reveal that ERK1 is dispensable for BRAFV600E-triggered cytotoxicity in lungs and that ERK-chemical inhibition abrogates some of these detrimental effects, such as DNA damage, in Club cells but not in pulmonary lymphocytes. Our data suggest that ERK1/ERK2 contribution to BRAFV600E-driven phenotypes is dynamic and varies dependently on cell type, the biological function, and the level of ERK-pathway activation. Our findings also provide useful insights into the comprehension of BRAFV600E-driven malignancies pathophysiology as well as the consequences in vivo of novel ERK pathway-targeted anti-cancer therapies.
© 2024. The Author(s).
-
IHC
-
Mus musculus (House mouse)
-
Cell Biology
In PLoS One on 15 July 2016 by Cox, A. R., Barrandon, O., et al.
Fig.9.C
-
WB
-
Mus musculus (House mouse)
Collected and cropped from PLoS ONE by CiteAb, provided under a CC-BY license
Image 1 of 1