T cell expansion has a crucial function in both autoimmune and chronic inflammatory diseases, with cycling T cells contributing to the pathogenesis of autoimmune diseases by causing uncontrolled immune responses and tissue damage. Yet the regulatory mechanisms governing T cell expansion remain incompletely understood. Here we show that the enzyme N-acetyltransferase 10 (NAT10) regulates T cell activation and proliferation upon antigen stimulation. T cell-specific NAT10 deficiency in mice reduces the number of mature T cells in peripheral lymphoid organs. Mechanistically, NAT10 acetylates RACK1 at K185, preventing subsequent RACK1 K48-linked ubiquitination and degradation. The increased RACK1 stability alters ribosome formation and cellular metabolism, leading to enhanced supply of energy and biosynthetic precursors and, eventually, T cell proliferation. Our findings thus highlight the essential function of NAT10 in T cell self-renewal and metabolism and elucidate NAT10 mode of action for the potential development of novel therapies for immune-related disorders.
© 2024. The Author(s).
Product Citations: 118
In Nature Communications on 30 October 2024 by Li, W. P., Mao, X. T., et al.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
TLR9 Monotherapy in Immune-Competent Mice Suppresses Orthotopic Prostate Tumor Development.
In Cells on 2 January 2024 by Miles, M. A., Luong, R., et al.
Prostate cancer is ranked second in the world for cancer-related deaths in men, highlighting the lack of effective therapies for advanced-stage disease. Toll-like receptors (TLRs) and immunity have a direct role in prostate cancer pathogenesis, but TLR9 has been reported to contribute to both the progression and inhibition of prostate tumorigenesis. To further understand this apparent disparity, we have investigated the effect of TLR9 stimulation on prostate cancer progression in an immune-competent, syngeneic orthotopic mouse model of prostate cancer. Here, we utilized the class B synthetic agonist CPG-1668 to provoke a TLR9-mediated systemic immune response and demonstrate a significant impairment of prostate tumorigenesis. Untreated tumors contained a high abundance of immune-cell infiltrates. However, pharmacological activation of TLR9 resulted in smaller tumors containing significantly fewer M1 macrophages and T cells. TLR9 stimulation of tumor cells in vitro had no effect on cell viability or its downstream transcriptional targets, whereas stimulation in macrophages suppressed cancer cell growth via type I IFN. This suggests that the antitumorigenic effects of CPG-1668 were predominantly mediated by an antitumor immune response. This study demonstrated that systemic TLR9 stimulation negatively regulates prostate cancer tumorigenesis and highlights TLR9 agonists as a useful therapeutic for the treatment of prostate cancer.
-
FC/FACS
-
Mus musculus (House mouse)
-
Cancer Research
-
Cell Biology
-
Immunology and Microbiology
In EBioMedicine on 1 January 2024 by O'Neill, A., Mantri, C. K., et al.
COVID-19 vaccines used in humans are highly effective in limiting disease and death caused by the SARS-CoV-2 virus, yet improved vaccines that provide greater protection at mucosal surfaces, which could reduce break-through infections and subsequent transmission, are still needed.
Here we tested an intranasal (I.N.) vaccination with the receptor binding domain of Spike antigen of SARS-CoV-2 (S-RBD) in combination with the mucosal adjuvant mastoparan-7 compared with the sub-cutaneous (S.C.) route, adjuvanted by either M7 or the gold-standard adjuvant, alum, in mice, for immunological read-outs. The same formulation delivered I.N. or S.C. was tested in hamsters to assess efficacy.
I.N. vaccination improved systemic T cell responses compared to an equivalent dose of antigen delivered S.C. and T cell phenotypes induced by I.N. vaccine administration included enhanced polyfunctionality (combined IFN-γ and TNF expression) and greater numbers of T central memory (TCM) cells. These phenotypes were T cell-intrinsic and could be recalled in the lungs and/or brachial LNs upon antigen challenge after adoptive T cell transfer to naïve recipients. Furthermore, mucosal vaccination induced antibody responses that were similarly effective in neutralising the binding of the parental strain of S-RBD to its ACE2 receptor, but showed greater cross-neutralising capacity against multiple variants of concern (VOC), compared to S.C. vaccination. I.N. vaccination provided significant protection from lung pathology compared to unvaccinated animals upon challenge with homologous and heterologous SARS-CoV-2 strains in a hamster model.
These results highlight the role of nasal vaccine administration in imprinting an immune profile associated with long-term T cell retention and diversified neutralising antibody responses, which could be applied to improve vaccines for COVID-19 and other infectious diseases.
This study was funded by Duke-NUS Medical School, the Singapore Ministry of Education, the National Medical Research Council of Singapore and a DBT-BIRAC Grant.
Copyright © 2023 The Author(s). Published by Elsevier B.V. All rights reserved.
-
Mus musculus (House mouse)
-
COVID-19
The HDAC inhibitor zabadinostat is a systemic regulator of adaptive immunity.
In Communications Biology on 26 January 2023 by Liu, G., Barczak, W., et al.
Protein acetylation plays a key role in regulating cellular processes and is subject to aberrant control in diverse pathologies. Although histone deacetylase (HDAC) inhibitors are approved drugs for certain cancers, it is not known whether they can be deployed in other therapeutic contexts. We have explored the clinical HDAC inhibitor, zabadinostat/CXD101, and found that it is a stand-alone regulator of the adaptive immune response. Zabadinostat treatment increased expression of MHC class I and II genes in a variety of cells, including dendritic cells (DCs) and healthy tissue. Remarkably, zabadinostat enhanced the activity of DCs, and CD4 and CD8 T lymphocytes. Using an antigenic peptide presented to the immune system by MHC class I, zabadinostat caused an increase in antigen-specific CD8 T lymphocytes. Further, mice immunised with covid19 spike protein and treated with zabadinostat exhibit enhanced covid19 neutralising antibodies and an increased level of T lymphocytes. The enhanced humoral response reflected increased activity of T follicular helper (Tfh) cells and germinal centre (GC) B cells. Our results argue strongly that zabadinostat has potential to augment diverse therapeutic agents that act through the immune system.
© 2023. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Cell Reports on 1 November 2022 by Deák, P., Studnitzer, B., et al.
Dendritic cell (DC) activation via pathogen-associated molecular patterns (PAMPs) is critical for antigen presentation and development of adaptive immune responses, but the stochastic distribution of DC responses to PAMP signaling, especially during the initial stages of immune activation, is poorly understood. In this study, we isolate a unique DC subpopulation via preferential phagocytosis of microparticles (MPs) and characterize this subpopulation of "first responders" (FRs). We present results that show these cells (1) can be isolated and studied via both increased accumulation of the micron-sized particles and combinations of cell surface markers, (2) show increased responses to PAMPs, (3) facilitate adaptive immune responses by providing the initial paracrine signaling, and (4) can be selectively targeted by vaccines to modulate both antibody and T cell responses in vivo. This study presents insights into a temporally controlled, distinctive cell population that influences downstream immune responses. Furthermore, it demonstrates potential for improving vaccine designs via FR targeting.Copyright © 2022. Published by Elsevier Inc.
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Cells on 2 January 2024 by Miles, M. A., Luong, R., et al.
Fig.4.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Cells by CiteAb, provided under a CC-BY license
Image 1 of 5
In Cells on 2 January 2024 by Miles, M. A., Luong, R., et al.
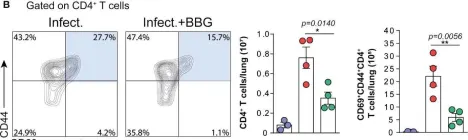
Fig.2.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Cells by CiteAb, provided under a CC-BY license
Image 1 of 5
In Front Cell Infect Microbiol on 25 May 2021 by Santiago-Carvalho, I., de Almeida-Santos, G., et al.
Fig.4.B

-
FC/FACS
-
Collected and cropped from Front Cell Infect Microbiol by CiteAb, provided under a CC-BY license
Image 1 of 5
In Nat Commun on 19 December 2019 by McCarron, M. J., Irla, M., et al.
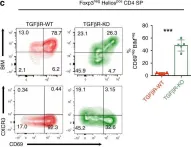
Fig.5.C

-
FC/FACS
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 5
In Elife on 19 July 2016 by Raguz, J., Jerić, I., et al.
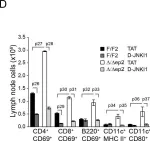
Fig.8.D

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 5