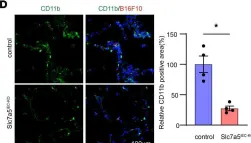
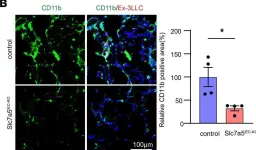
Some endothelial cells in the tumor vasculature express a system L amino acid transporter, LAT1. To elucidate the role of LAT1 in tumor-related endothelial cells, tumor cells were injected into endothelial cell-specific LAT1 conditional knockout mice (Slc7a5flox/flox; Cdh5-Cre-ERT2), and we found that the shape of the tumor vasculature was normalized and the size and numbers of lung metastasis was reduced. TNF-α-induced expression of VCAM1 and E-selectin at the surface of HUVEC, both of which are responsible for enhanced monocyte attachment and premetastatic niche formation, was reduced in the presence of LAT1 inhibitor, nanvuranlat. Deprivation of tryptophan, a LAT1 substrate, mimicked LAT1 inhibition, which led to activation of MEK1/2-ERK1/2 pathway and subsequent cystathionine γ lyase (CTH) induction. Increased production of hydrogen sulfide (H2S) by CTH was at least partially responsible for tumor vascular normalization, leading to decreased leakiness and enhanced delivery of chemotherapeutic agents to the tumor.
Product Citations: 13
Endothelial cell-specific LAT1 ablation normalizes tumor vasculature.
In JCI Insight on 20 August 2024 by Suehiro, J. I., Kimura, T., et al.
-
IHC-IF
-
Cancer Research
Oligodendroglia-to-pericyte conversion after lipopolysaccharide exposure is gender-dependent.
In PLoS ONE on 6 August 2024 by Yu, Q., Zhang, L., et al.
To investigate the sex-dependent differentiation of Sox10 cells and their response to pathological conditions such as lipopolysaccharide (LPS) exposure or ischemia, we utilized Sox10 Cre-ERT2, tdTomato mice. Tamoxifen administration induced the expression of red fluorescent protein (RFP) in these cells, facilitating their subsequent tracking and analysis after LPS injection and ischemia via immunofluorescence staining. Propidium iodide (PI) was injected to label necrotic cells following LPS administration. We found that the conversion of Sox10 cells to pericytes in female mice was significantly higher than in male mice, especially in those exposed to LPS. After LPS injection, the number of PI+ necrotic cells were significantly greater in females than in males. Moreover, RFP+ cells did not co-localize with glial fibrillary acidic protein (GFAP) or cluster of differentiation 11b (CD11b). Similarly, after brain ischemia, RFP+ cells did not express cluster of differentiation 13 (CD13), neuronal nuclei (NeuN), GFAP, or ionised calcium binding adaptor molecule 1 (Iba-1). These findings indicate that the conversion of Sox10 cells to pericytes following LPS exposure is sex-dependent, with neither male nor female groups showing differentiation into other cell types after LPS exposure or under ischemic conditions. The differences in LPS-induced necrosis of pericytes between sexes may explain the variations in the conversion of Sox10 cells to pericytes in both sexes.
Copyright: © 2024 Yu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
-
IHC-IF
In Nature Communications on 13 June 2023 by Lenza, M. P., Egia-Mendikute, L., et al.
Sialic acid-binding Ig-like lectin 15 (Siglec-15) is an immune modulator and emerging cancer immunotherapy target. However, limited understanding of its structure and mechanism of action restrains the development of drug candidates that unleash its full therapeutic potential. In this study, we elucidate the crystal structure of Siglec-15 and its binding epitope via co-crystallization with an anti-Siglec-15 blocking antibody. Using saturation transfer-difference nuclear magnetic resonance (STD-NMR) spectroscopy and molecular dynamics simulations, we reveal Siglec-15 binding mode to α(2,3)- and α(2,6)-linked sialic acids and the cancer-associated sialyl-Tn (STn) glycoform. We demonstrate that binding of Siglec-15 to T cells, which lack STn expression, depends on the presence of α(2,3)- and α(2,6)-linked sialoglycans. Furthermore, we identify the leukocyte integrin CD11b as a Siglec-15 binding partner on human T cells. Collectively, our findings provide an integrated understanding of the structural features of Siglec-15 and emphasize glycosylation as a crucial factor in controlling T cell responses.
© 2023. The Author(s).
-
Homo sapiens (Human)
-
Immunology and Microbiology
A single dose of lipopolysaccharide elicits autofluorescence in the mouse brain.
In Frontiers in Aging Neuroscience on 7 April 2023 by Yang, Y., Yu, Q., et al.
One hallmark of aging is autofluorescence (AF) in the brain. However, the underlying mechanism for inducing AF remains unknown. This study aims to determine the cause(s) of this phenomenon. The endogenous expression pattern of AF in mice was examined at differing ages. Intraperitoneal injection of a single dose of lipopolysaccharide (LPS) was performed to induce AF. Copper sulfate was applied to remove AF to allow for further immunofluorescence staining. AF appeared in the mouse brain as early as 3 months of age. In the cortex, AF occurs in the lysosomes of microglia, astrocytes, endothelial cells, and oligodendrocyte lineage cells and its prevalence increases with age. Interestingly, AF never occurs in the pericytes of young or aged brains. LPS administration resulted in a rapid and marked induction of brain AF, similar to the normal aging process. Finally, age-related and induced AF can be eliminated by low concentrations of copper sulfate solution. This pre-treatment is safe for aging and lineage tracing studies. These findings depict that AF in the brain could be associated with the innate immune response against Gram-negative bacteria infection.
Copyright © 2023 Yang, Yu, Li, Li, Yang, Yuan and Liu.
-
IHC
-
Mus musculus (House mouse)
In Cell Stem Cell on 1 December 2022 by Porpiglia, E., Mai, T., et al.
In aging, skeletal muscle strength and regenerative capacity decline, due in part to functional impairment of muscle stem cells (MuSCs), yet the underlying mechanisms remain elusive. Here, we capitalize on mass cytometry to identify high CD47 expression as a hallmark of dysfunctional MuSCs (CD47hi) with impaired regenerative capacity that predominate with aging. The prevalent CD47hi MuSC subset suppresses the residual functional CD47lo MuSC subset through a paracrine signaling loop, leading to impaired proliferation. We uncover that elevated CD47 levels on aged MuSCs result from increased U1 snRNA expression, which disrupts alternative polyadenylation. The deficit in aged MuSC function in regeneration can be overcome either by morpholino-mediated blockade of CD47 alternative polyadenylation or antibody blockade of thrombospondin-1/CD47 signaling, leading to improved regeneration in aged mice, with therapeutic implications. Our findings highlight a previously unrecognized age-dependent alteration in CD47 levels and function in MuSCs, which underlies reduced muscle repair in aging.Copyright © 2022 Elsevier Inc. All rights reserved.
-
Mus musculus (House mouse)
-
Stem Cells and Developmental Biology
In JCI Insight on 20 August 2024 by Suehiro, J. I., Kimura, T., et al.
Fig.4.D

-
IHC-IF
-
Collected and cropped from JCI Insight by CiteAb, provided under a CC-BY license
Image 1 of 3
In JCI Insight on 20 August 2024 by Suehiro, J. I., Kimura, T., et al.
Fig.4.I

-
IHC-IF
-
Collected and cropped from JCI Insight by CiteAb, provided under a CC-BY license
Image 1 of 3
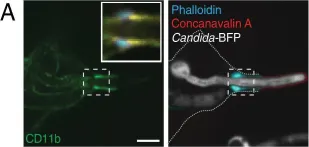
In Elife on 19 March 2018 by Maxson, M. E., Naj, X., et al.
Fig.3.A

-
ICC-IF
-
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 3