The estrogen receptor (ER) designated ERα has actions in many cell and tissue types that impact glucose homeostasis. It is unknown if these include mechanisms in endothelial cells, which have the potential to influence relative obesity, and processes in adipose tissue and skeletal muscle that impact glucose control. Here we show that independent of impact on events in adipose tissue, endothelial ERα promotes glucose tolerance by enhancing endothelial insulin transport to skeletal muscle. Endothelial ERα-deficient male mice are glucose intolerant and insulin resistant, and in females the antidiabetogenic actions of estradiol (E2) are absent. The glucose dysregulation is due to impaired skeletal muscle glucose disposal that results from attenuated muscle insulin delivery. Endothelial ERα activation stimulates insulin transcytosis by skeletal muscle microvascular endothelial cells. Mechanistically this involves nuclear ERα-dependent upregulation of vesicular trafficking regulator sorting nexin 5 (SNX5) expression, and PI3 kinase activation that drives plasma membrane recruitment of SNX5. Thus, coupled nuclear and non-nuclear actions of ERα promote endothelial insulin transport to skeletal muscle to foster normal glucose homeostasis.
© 2023. Springer Nature Limited.
Product Citations: 112
In Nature Communications on 17 August 2023 by Sacharidou, A., Chambliss, K., et al.
-
Endocrinology and Physiology
In Nature Communications on 25 July 2023 by Rondeaux, J., Groussard, D., et al.
Epigenetic regulation of histone H3K27 methylation has recently emerged as a key step during alternative immunoregulatory M2-like macrophage polarization; known to impact cardiac repair after Myocardial Infarction (MI). We hypothesized that EZH2, responsible for H3K27 methylation, could act as an epigenetic checkpoint regulator during this process. We demonstrate for the first time an ectopic EZH2, and putative, cytoplasmic inactive localization of the epigenetic enzyme, during monocyte differentiation into M2 macrophages in vitro as well as in immunomodulatory cardiac macrophages in vivo in the post-MI acute inflammatory phase. Moreover, we show that pharmacological EZH2 inhibition, with GSK-343, resolves H3K27 methylation of bivalent gene promoters, thus enhancing their expression to promote human monocyte repair functions. In line with this protective effect, GSK-343 treatment accelerated cardiac inflammatory resolution preventing infarct expansion and subsequent cardiac dysfunction in female mice post-MI in vivo. In conclusion, our study reveals that pharmacological epigenetic modulation of cardiac-infiltrating immune cells may hold promise to limit adverse cardiac remodeling after MI.
© 2023. The Author(s).
-
Cardiovascular biology
-
Genetics
In American Journal of Cancer Research on 9 June 2023 by Huang, S., Xie, P., et al.
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer-related mortality globally with limited effective treatment options. Although the combination of immunotherapy and chemotherapy has been attempted in clinical trials to treat PDAC, the results are not promising. Therefore, in this study, we explored the application of a novel combination strategy with disulfiram (DSF) to enhance the treatment efficacy of PDAC as well as its underlying molecular mechanism. We compared the antitumor effects between single agents and the combination therapy by using mouse allograft tumor model and found DSF combined with chemoimmunotherapy significantly suppressed the growth of subcutaneous PDAC allograft tumor in mice and prolonged the survival of mice. To further investigate the alterations in the immune microenvironment of tumors from different treatment groups, we employed flow cytometry and RNA-seq analysis to examine the composition of tumor-infiltrating immune cells as well as the expression level of a variety of cytokines. Our results revealed that the proportion of CD8 T cells was notably elevated and that multiple cytokines were upregulated in the combination therapy group. Furthermore, qRT-PCR results indicated that DSF could upregulate the mRNA levels of IFNα and IFNβ, which could be reversed by STING pathway inhibitor. Mechanistically, we found that DSF activated STING signaling pathway through Poly (ADP-ribose) polymerases (PARP1) inhibition. Taken together, our findings highlight the potential clinical application of this novel combination strategy using DSF and chemoimmunotherapy in the treatment of patients with PDAC.
AJCR Copyright © 2023.
-
Cancer Research
Preprint on BioRxiv : the Preprint Server for Biology on 3 January 2023 by Wang, J., Cheng, M., et al.
Pigs are the most suitable model to study various therapeutic strategies and drugs for human beings, while knowledge about tissue- and cell type-specific transcriptomes and heterogeneity is poorly available. Here, we focused on the intestinal immunity of pigs. Through single-cell sequencing (scRNA-seq) and flow cytometry analysis of the types of immune cells in the jejunum of pigs, we found that innate lymphoid cells (ILCs) existed in the lamina propria lymphocytes (LPLs) of the jejunum. Then, through flow sorting of Live/Dead (L/D) - Lineage(LIN) - CD45 + cells and scRNA-seq, we found that ILCs in the porcine jejunum were mainly ILC3s, with a small number of ILC1s, ILC2s and NK cells. Through a gene expression map, we found that ILCs coexpressed IL-7Rα, ID2 and other genes and differentially expressed RORC, GATA3 and other genes but did not express the CD3 gene. According to their gene expression profiles, ILC3s can be divided into four subgroups, and genes such as CXCL8, CXCL2, IL-22, IL-17 and NCR2 are differentially expressed. To further detect and identify ILC3s, we prepared RORC monoclonal antibodies and verified the classification of ILCs in the porcine jejunum subgroup and the expression of related hallmark genes at the protein level by flow cytometry. For systematically characterizing of ILCs in the porcin intestines, we combined our pigs ILCs dataset with publicly available humans and mice ILCs data and identified that the humans and pigs ILCs shared more common features than that of mice in gene signatures and cell states. Our results for the first time showed in detail the gene expression of porcine jejunal ILCs, the subtype classification of ILCs and the markers of various ILCs, which provides a basis for in-depth exploration of porcine intestinal mucosal immunity. Graphical abstract
-
Biochemistry and Molecular biology
-
Veterinary Research
In IScience on 21 October 2022 by Roy, I. M., Anu, P. V., et al.
Interaction with microenvironmental factors is crucial for the regulation of hematopoietic stem cell (HSC) function. Stroma derived factor (SDF)-1α supports HSCs in the quiescent state and is central to the homing of transplanted HSCs. Here, we show that integrin signaling regulates Sdf-1α expression transcriptionally. Systemic deletion of Periostin, an Integrin-αv ligand, showed increased expression of Sdf-1α in bone marrow (BM) niche. Pharmacological inhibition or CRISPR-Cas9-mediated deletion of SRC, resulted in a similar increase in the chemokine expression in vitro. Importantly, systemic SRC-inhibition led to increase in SDF-1α levels in BM plasma. This resulted in a robust increase (14.05 ± 1.22% to 29.11 ± 0.69%) in the homing efficiency of transplanted HSCs. In addition, we observed enhancement in the recovery of blood cell counts following radiation injury, indicating an enhanced hematopoietic function. These results establish a role of SRC-mediated integrin signaling in the transcriptional regulation of Sdf-1α. This mechanism could be harnessed further to improve the hematopoietic function.
© 2022 The Authors.
-
Stem Cells and Developmental Biology
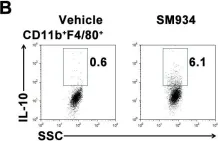
In PLoS One on 6 March 2012 by Hou, L. F., He, S. J., et al.
Fig.6.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 2
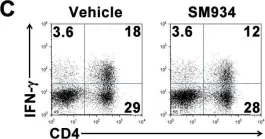
In PLoS One on 6 March 2012 by Hou, L. F., He, S. J., et al.
Fig.6.C

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 2