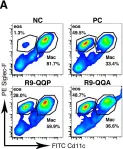
Osteosarcoma (OS) with pulmonary metastasis remains challenging due to limited treatment options and the immunosuppressive nature of the tumor microenvironment (TME). Bacteria-mediated cancer therapy has emerged as a promising strategy for solid tumors but often suffers from limited efficacy due to the immunosuppressive TME, which restricts the intensity and durability of the antitumor immune response. To overcome these challenges, we engineered a novel Salmonella strain, VNP20009-CCL2-CXCL9 (VNP-C-C), leveraging the intrinsic tumor tropism of Salmonella typhimurium VNP20009 (VNP) and improving immune modulation through the recruitment of effector immune cells into the TME by the chemokines CCL2 and CXCL9.
VNP-C-C was genetically engineered through electroporation of Plac-CCL2-CXCL9 plasmid and validated in vitro. Its antitumor efficacy, immune regulation capacity and immunomodulatory mechanisms were evaluated in vitro by using OS cell lines and immune cells (dendritic cells (DCs) and macrophages (Mφs)) and in vivo by using both immunocompromised and immunocompetent mouse models of OS lung metastasis.
VNP-C-C effectively accumulated within tumors, triggering immunogenic cell death and subsequently activating the cyclic GMP-AMP synthase (cGAS)/stimulator of interferon genes (STING) pathway, thereby robustly promoting type I interferon secretion. The chemokines CCL2 and CXCL9 amplified the immune response by recruiting DCs, Mφs, and T cells to the TME. This orchestrated immune modulation reprogrammed tumor-associated macrophages to an antitumor phenotype, induced DCs maturation, significantly increased T-cell infiltration and activation within tumors, and promoted systemic T-cell memory formation in peripheral lymphoid organs. These effects collectively inhibited OS lung metastasis progression and provided survival benefits in mouse models.
The engineered bacterial strain VNP-C-C effectively converts the OS lung metastatic TME into a pro-inflammatory milieu, thereby stimulating robust innate and adaptive immune responses. This offers a highly promising therapeutic avenue for OS lung metastasis with considerable translational potential in cancer immunotherapy.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Product Citations: 59
In Journal for Immunotherapy of Cancer on 1 July 2025 by Liu, R., Liu, Q., et al.
-
FC/FACS
-
Mus musculus (House mouse)
-
Cancer Research
-
Immunology and Microbiology
In PLoS Pathogens on 1 February 2025 by Bercusson, A., Williams, T. J., et al.
Aspergillus fumigatus (Af) is a major mould pathogen found ubiquitously in the air. It commonly infects the airways of people with cystic fibrosis (CF) leading to Aspergillus bronchitis or allergic bronchopulmonary aspergillosis. Resident alveolar macrophages and recruited neutrophils are important first lines of defence for clearance of Af in the lung. However, their contribution to the inflammatory phenotype in CF during Af infection is not well understood. Here, utilising CFTR deficient mice we describe a hyperinflammatory phenotype in both acute and allergic murine models of pulmonary aspergillosis. We show that during aspergillosis, CFTR deficiency leads to increased alveolar macrophage death and persistent inflammation of the airways in CF, accompanied by impaired fungal control. Utilising CFTR deficient murine cells and primary human CF cells we show that at a cellular level there is increased activation of NFκB and NFAT in response to Af which, as in in vivo models, is associated with increased cell death and reduced fungal control. Taken together, these studies indicate that CFTR deficiency promotes increased activation of inflammatory pathways, the induction of macrophage cell death and reduced fungal control contributing to the hyper-inflammatory of pulmonary aspergillosis phenotypes in CF.
Copyright: © 2025 Bercusson et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
-
Immunology and Microbiology
Preprint on BioRxiv : the Preprint Server for Biology on 3 December 2024 by Bercusson, A., Williams, T. J., et al.
Aspergillus fumigatus ( Af ) is a major mould pathogen found ubiquitously in the air. It commonly infects the airways of people with cystic fibrosis (CF) leading to Aspergillus bronchitis or allergic bronchopulmonary aspergillosis. Resident alveolar macrophages and recruited neutrophils are important first lines of defence for clearance of Af in the lung. However, their contribution to the inflammatory phenotype in CF during Af infection is not well understood. Here, utilising CFTR deficient mice we describe a hyperinflammatory phenotype in both acute and allergic murine models of pulmonary aspergillosis. We show that during aspergillosis, CFTR deficiency leads to increased alveolar macrophage death and persistent inflammation of the airways in CF, accompanied by impaired fungal control. Utilising CFTR deficient murine cells and primary human CF cells we show that at a cellular level there is increased activation of NFκB and NFAT in response to Af which, as in in vivo models, is associated with increased cell death and reduced fungal control. Taken together, these studies indicate that CFTR deficiency promotes increased activation of inflammatory pathways, the induction of macrophage cell death and reduced fungal control contributing to the hyper-inflammatory of pulmonary aspergillosis phenotypes in CF. Author Summary The airways of people with cystic fibrosis (pwCF) are commonly colonised with Aspergillus fumigatus ( Af ) resulting in a persistent hyperinflammatory state and the development of allergy. Understanding how first line defence innate cells, such as alveolar macrophages and neutrophils, contribute to this hyperinflammatory phenotype is important in developing novel treatments to preserve lung function in pwCF. In this study, we report that CFTR deficiency leads to increased alveolar macrophage death and persistent inflammation of the airways in pwCF, which is associated with impaired control of infection. We identify the increased activation of the transcription factors NFκB and NFAT as the mechanism of increased inflammatory cytokine production in CFTR deficient cells. This work is the first step in describing molecular mechanisms of hyperinflammation in CF in response to fungal infection and lays the groundwork for further dissection of inflammatory pathways to target with immunotherapeutic approaches.
-
Mus musculus (House mouse)
Cell state dependent effects of Bmal1 on melanoma immunity and tumorigenicity.
In Nature Communications on 20 January 2024 by Zhang, X., Pant, S. M., et al.
The circadian clock regulator Bmal1 modulates tumorigenesis, but its reported effects are inconsistent. Here, we show that Bmal1 has a context-dependent role in mouse melanoma tumor growth. Loss of Bmal1 in YUMM2.1 or B16-F10 melanoma cells eliminates clock function and diminishes hypoxic gene expression and tumorigenesis, which could be rescued by ectopic expression of HIF1α in YUMM2.1 cells. By contrast, over-expressed wild-type or a transcriptionally inactive mutant Bmal1 non-canonically sequester myosin heavy chain 9 (Myh9) to increase MRTF-SRF activity and AP-1 transcriptional signature, and shift YUMM2.1 cells from a Sox10high to a Sox9high immune resistant, mesenchymal cell state that is found in human melanomas. Our work describes a link between Bmal1, Myh9, mouse melanoma cell plasticity, and tumor immunity. This connection may underlie cancer therapeutic resistance and underpin the link between the circadian clock, MRTF-SRF and the cytoskeleton.
© 2024. The Author(s).
-
Mus musculus (House mouse)
-
Cancer Research
-
Immunology and Microbiology
In Cell Reports Medicine on 16 January 2024 by Wallace, R. P., Refvik, K. C., et al.
Immunogenic biologics trigger an anti-drug antibody (ADA) response in patients that reduces efficacy and increases adverse reactions. Our laboratory has shown that targeting protein antigen to the liver microenvironment can reduce antigen-specific T cell responses; herein, we present a strategy to increase delivery of otherwise immunogenic biologics to the liver via conjugation to a synthetic mannose polymer, p(Man). This delivery leads to reduced antigen-specific T follicular helper cell and B cell responses resulting in diminished ADA production, which is maintained throughout subsequent administrations of the native biologic. We find that p(Man)-antigen treatment impairs the ADA response against recombinant uricase, a highly immunogenic biologic, without a dependence on hapten immunodominance or control by T regulatory cells. We identify increased T cell receptor signaling and increased apoptosis and exhaustion in T cells as effects of p(Man)-antigen treatment via transcriptomic analyses. This modular platform may enhance tolerance to biologics, enabling long-term solutions for an ever-increasing healthcare problem.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In PLoS One on 15 May 2013 by Guimond, D. M., Cam, N. R., et al.
Fig.5.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 1