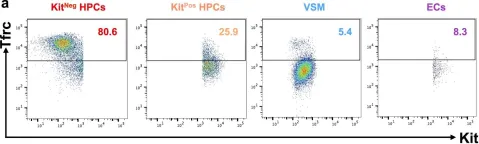
In this study, we aimed to explore how cellular iron status affects embryonic haematopoiesis. For this purpose, we used a model of mouse embryonic stem cell differentiation into embryonic haematopoietic progenitors. We modulated the iron status by adding either the iron chelator Deferoxamine (DFO) for iron deficiency, or ferric ammonium citrate for iron excess, and followed the emergence of developing haematopoietic progenitors. Interestingly, we found that iron deficiency did not block the endothelial to haematopoietic transition, the first step of haematopoiesis. However, it did reduce the proliferation, survival and clonogenic capacity of haematopoietic progenitors. Surprisingly, iron deficiency affected erythro-myeloid progenitors significantly more than the primitive erythroid ones. Erythro-myeloid progenitors expressed less transferrin-receptor on the cell surface and had less labile iron compared to primitive erythroid progenitors, which could reduce their capacity to compete for scarce iron and survive iron deficiency. In conclusion, we show that iron deficiency could disturb haematopoiesis at an early embryonic stage by compromising more severely the survival, proliferation and differentiation of definitive haematopoietic progenitors compared to restricted erythroid progenitors.
Product Citations: 7
In Scientific Reports on 23 April 2019 by Shvartsman, M., Bilican, S., et al.
-
FC/FACS
-
Mus musculus (House mouse)
-
Stem Cells and Developmental Biology
Global increase in replication fork speed during a p57KIP2-regulated erythroid cell fate switch.
In Science Advances on 1 May 2017 by Hwang, Y., Futran, M., et al.
Cell cycle regulators are increasingly implicated in cell fate decisions, such as the acquisition or loss of pluripotency and self-renewal potential. The cell cycle mechanisms that regulate these cell fate decisions are largely unknown. We studied an S phase-dependent cell fate switch, in which murine early erythroid progenitors transition in vivo from a self-renewal state into a phase of active erythroid gene transcription and concurrent maturational cell divisions. We found that progenitors are dependent on p57KIP2-mediated slowing of replication forks for self-renewal, a novel function for cyclin-dependent kinase inhibitors. The switch to differentiation entails rapid down-regulation of p57KIP2 with a consequent global increase in replication fork speed and an abruptly shorter S phase. Our work suggests that cell cycles with specialized global DNA replication dynamics are integral to the maintenance of specific cell states and to cell fate decisions.
-
FC/FACS
-
Mus musculus (House mouse)
-
Stem Cells and Developmental Biology
In Blood on 12 July 2012 by Roth, M., Will, B., et al.
Eltrombopag (EP) is a small-molecule, nonpeptide thrombopoietin receptor (TPO-R) agonist that has been approved recently for the treatment of thrombocytopenia in patients with chronic immune thrombocytopenic purpura. Prior studies have shown that EP stimulates megakaryopoiesis in BM cells from patients with acute myeloid leukemia and myelodysplastic syndrome, and the results also suggested that it may inhibit leukemia cell growth. In the present study, we studied the effects of EP on leukemia cell proliferation and the mechanism of its antiproliferative effects. We found that EP leads to a decreased cell division rate, a block in G(1) phase of cell cycle, and increased differentiation in human and murine leukemia cells. Because EP is species specific in that it can only bind TPO-R in human and primate cells, these findings further suggested that the antileukemic effect is independent of TPO-R. We found that treatment with EP leads to a reduction in free intracellular iron in leukemic cells in a dose-dependent manner. Experimental increase of intracellular iron abrogated the antiproliferative and differentiation-inducing effects of EP, demonstrating that its antileukemic effects are mediated through modulation of intracellular iron content. Finally, determination of EP's antileukemic activity in vivo demonstrated its ability to prolong survival in 2 mouse models of leukemia.
-
FC/FACS
-
Homo sapiens (Human)
-
Mus musculus (House mouse)
-
Cancer Research
-
Cardiovascular biology
Notch3 is dispensable for thymocyte β-selection and Notch1-induced T cell leukemogenesis.
In PLoS ONE on 21 September 2011 by Suliman, S., Tan, J., et al.
Notch1 (N1) signaling induced by intrathymic Delta-like (DL) ligands is required for T cell lineage commitment as well as self-renewal during "β-selection" of TCRβ⁺CD4⁻CD8⁻ double negative 3 (DN3) T cell progenitors. However, over-expression of the N1 intracellular domain (ICN1) renders N1 activation ligand-independent and drives leukemic transformation during β-selection. DN3 progenitors also express Notch3 (N3) mRNA, and over-expression of ligand-independent mutant N3 (ICN3) influences β-selection and drives T cell leukemogenesis. However, the importance of ligand-activated N3 in promoting β-selection and ICN1-induced T cell leukemogenesis has not been examined. To address these questions we generated mice lacking functional N3. We confirmed that DN3 progenitors express N3 protein using a N3-specific antibody. Surprisingly however, N3-deficient DN3 thymocytes were not defective in generating DP thymocytes under steady state conditions or in more stringent competition assays. To determine if N3 co-operates with N1 to regulate β-selection, we generated N1;N3 compound mutants. However, N3 deficiency did not exacerbate the competitive defect of N1⁺/⁻ DN3 progenitors, demonstrating that N3 does not compensate for limiting N1 during T cell development. Finally, N3 deficiency did not attenuate T cell leukemogenesis induced by conditional expression of ICN1 in DN3 thymocytes. Importantly, we showed that in contrast to N1, N3 has a low binding affinity for DL4, the most abundant intrathymic DL ligand. Thus, despite the profound effects of ectopic ligand-independent N3 activation on T cell development and leukemogenesis, physiologically activated N3 is dispensable for both processes, likely because N3 interacts poorly with intrathymic DL4.
-
Immunology and Microbiology
In Developmental Cell on 1 October 2009 by Maeda, T., Ito, K., et al.
GATA-1-dependent transcription is essential for erythroid differentiation and maturation. Suppression of programmed cell death is also thought to be critical for this process; however, the link between these two features of erythropoiesis has remained elusive. Here, we show that the POZ-Krüppel family transcription factor, LRF (also known as Zbtb7a/Pokemon), is a direct target of GATA1 and plays an essential antiapoptotic role during terminal erythroid differentiation. We find that loss of Lrf leads to lethal anemia in embryos, due to increased apoptosis of late-stage erythroblasts. This programmed cell death is Arf and p53 independent and is instead mediated by upregulation of the proapoptotic factor Bim. We identify Lrf as a direct repressor of Bim transcription. In strong support of this mechanism, genetic Bim loss delays the lethality of Lrf-deficient embryos and rescues their anemia phenotype. Thus, our data define a key transcriptional cascade for effective erythropoiesis, whereby GATA-1 suppresses BIM-mediated apoptosis via LRF.
-
Stem Cells and Developmental Biology
In Sci Rep on 23 April 2019 by Shvartsman, M., Bilican, S., et al.
Fig.7.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 1