Schistosomiasis affects over 250 million people, predominantly in Sub-Saharan Africa. In Schistosoma -endemic regions a lack of natural sterilizing immunity means individuals are repeatedly infected, treated and reinfected. Due to difficulties in tracking infection in endemic areas, the kinetics of the host immune response during these reinfections have not been elucidated. Here, we use repeated (3x) controlled human Schistosoma mansoni ( Sm ) infection (repeated CHI) to directly study how antigen-specific T cells develop during reinfection. We compared these responses to natural Sm -infected endemic Ugandan individuals. We report that a mixed Th1/Th2/regulatory CD4 + T cell response develops in repeated CHI, primarily against adult worm antigens. Adult worm-specific responses after repeated CHI were similar to those observed in naturally infected endemic participants. However, endemic participants showed differential responses to antigens from the egg and cercariae life-cycle stages. Repeated CHI (3x) with sequential exposure to Sm cercariae of different sexes (male-female-male) revealed an elevated CD4 + T cell cytokine response to adult worm and egg antigens. Our findings demonstrate that reinfection with single-sex schistosomes elicits adult worm-specific T cell cytokine responses that reflect endemic-natural infection, highlighting the translatability of the CHI model to natural infection in endemic settings. Overall, this study advances our understanding of how schistosome immune responses develop over reinfection cycles in the human host, thereby increasing our understanding of the immunology of natural schistosome (re)infection.
Product Citations: 87
T cell responses in repeated controlled human schistosome infection compared to natural exposure
Preprint on MedRxiv : the Preprint Server for Health Sciences on 17 February 2025 by Driciru, E., Koopman, J. P. R., et al.
-
Immunology and Microbiology
In Cell Reports Medicine on 19 November 2024 by Tay, T., Bommakanti, G., et al.
In cancer, chronic antigen stimulation drives effector T cells to exhaustion, limiting the efficacy of T cell therapies. Recent studies have demonstrated that epigenetic rewiring governs the transition of T cells from effector to exhausted states and makes a subset of exhausted T cells non-responsive to PD1 checkpoint blockade. Here, we describe an antigen-specific assay for T cell exhaustion that generates T cells phenotypically and transcriptionally similar to those found in human tumors. We perform a screen of human epigenetic regulators, identifying IKZF1 as a driver of T cell exhaustion. We determine that the IKZF1 degrader iberdomide prevents exhaustion by blocking chromatin remodeling at T cell effector enhancers and preserving the binding of AP-1, NF-κB, and NFAT. Thus, our study uncovers a role for IKZF1 as a driver of T cell exhaustion through epigenetic modulation, providing a rationale for the use of iberdomide in solid tumors to prevent T cell exhaustion.
Copyright © 2024. Published by Elsevier Inc.
-
Genetics
-
Immunology and Microbiology
CD28 Costimulation Augments CAR Signaling in NK Cells via the LCK/CD3ζ/ZAP70 Signaling Axis.
In Cancer Discovery on 4 October 2024 by Acharya, S., Basar, R., et al.
Multiple factors in the design of a chimeric antigen receptor (CAR) influence CAR T-cell activity, with costimulatory signals being a key component. Yet, the impact of costimulatory domains on the downstream signaling and subsequent functionality of CAR-engineered natural killer (NK) cells remains largely unexplored. Here, we evaluated the impact of various costimulatory domains on CAR-NK cell activity, using a CD70-targeting CAR. We found that CD28, a costimulatory molecule not inherently present in mature NK cells, significantly enhanced the antitumor efficacy and long-term cytotoxicity of CAR-NK cells both in vitro and in multiple xenograft models of hematologic and solid tumors. Mechanistically, we showed that CD28 linked to CD3ζ creates a platform that recruits critical kinases, such as lymphocyte-specific protein tyrosine kinase (LCK) and zeta-chain-associated protein kinase 70 (ZAP70), initiating a signaling cascade that enhances CAR-NK cell function. Our study provides insights into how CD28 costimulation enhances CAR-NK cell function and supports its incorporation in NK-based CARs for cancer immunotherapy. Significance: We demonstrated that incorporation of the T-cell-centric costimulatory molecule CD28, which is normally absent in mature natural killer (NK) cells, into the chimeric antigen receptor (CAR) construct recruits key kinases including lymphocyte-specific protein tyrosine kinase and zeta-chain-associated protein kinase 70 and results in enhanced CAR-NK cell persistence and sustained antitumor cytotoxicity.
©2024 American Association for Cancer Research.
-
Cancer Research
In Cell Reports Medicine on 16 July 2024 by Zhao, Y., Li, S., et al.
The efficacy of chemotherapy varies significantly among patients with gastric cancer (GC), and there is currently no effective strategy to predict chemotherapeutic outcomes. In this study, we successfully establish 57 GC patient-derived organoids (PDOs) from 73 patients with GC (78%). These organoids retain histological characteristics of their corresponding primary GC tissues. GC PDOs show varied responses to different chemotherapeutics. Through RNA sequencing, the upregulation of tumor suppression genes/pathways is identified in 5-fluorouracil (FU)- or oxaliplatin-sensitive organoids, whereas genes/pathways associated with proliferation and invasion are enriched in chemotherapy-resistant organoids. Gene expression biomarker panels, which could distinguish sensitive and resistant patients to 5-FU and oxaliplatin (area under the dose-response curve [AUC] >0.8), are identified. Moreover, the drug-response results in PDOs are validated in patient-derived organoids-based xenograft (PDOX) mice and are consistent with the actual clinical response in 91.7% (11/12) of patients with GC. Assessing chemosensitivity in PDOs can be utilized as a valuable tool for screening chemotherapeutic drugs in patients with GC.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
-
Cancer Research
PGE2 inhibits TIL expansion by disrupting IL-2 signalling and mitochondrial function.
In Nature on 1 May 2024 by Morotti, M., Grimm, A. J., et al.
Expansion of antigen-experienced CD8+ T cells is critical for the success of tumour-infiltrating lymphocyte (TIL)-adoptive cell therapy (ACT) in patients with cancer1. Interleukin-2 (IL-2) acts as a key regulator of CD8+ cytotoxic T lymphocyte functions by promoting expansion and cytotoxic capability2,3. Therefore, it is essential to comprehend mechanistic barriers to IL-2 sensing in the tumour microenvironment to implement strategies to reinvigorate IL-2 responsiveness and T cell antitumour responses. Here we report that prostaglandin E2 (PGE2), a known negative regulator of immune response in the tumour microenvironment4,5, is present at high concentrations in tumour tissue from patients and leads to impaired IL-2 sensing in human CD8+ TILs via the PGE2 receptors EP2 and EP4. Mechanistically, PGE2 inhibits IL-2 sensing in TILs by downregulating the IL-2Rγc chain, resulting in defective assembly of IL-2Rβ-IL2Rγc membrane dimers. This results in impaired IL-2-mTOR adaptation and PGC1α transcriptional repression, causing oxidative stress and ferroptotic cell death in tumour-reactive TILs. Inhibition of PGE2 signalling to EP2 and EP4 during TIL expansion for ACT resulted in increased IL-2 sensing, leading to enhanced proliferation of tumour-reactive TILs and enhanced tumour control once the cells were transferred in vivo. Our study reveals fundamental features that underlie impairment of human TILs mediated by PGE2 in the tumour microenvironment. These findings have therapeutic implications for cancer immunotherapy and cell therapy, and enable the development of targeted strategies to enhance IL-2 sensing and amplify the IL-2 response in TILs, thereby promoting the expansion of effector T cells with enhanced therapeutic potential.
© 2024. The Author(s).
-
FC/FACS
-
Homo sapiens (Human)
-
Cell Biology
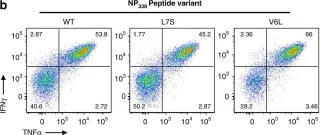
In Nat Commun on 21 December 2018 by Grant, E. J., Josephs, T. M., et al.
Fig.2.B

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 1