The use of programmed death-1 (PD-1) inhibitors in the neoadjuvant setting for patients with resectable stage III NSCLC has revolutionized this field in recent years. However, there is still 40%-60% of patients do not benefit from this approach. The complex interactions between immune cell subtypes and tertiary lymphoid structures (TLSs) within the tumor microenvironment (TME) may influence prognosis and the response to immunochemotherapy. This study aims to assess the relationship between immune cells subtypes and TLSs to better understand their impact on immunotherapy response.
This study initially compared the tertiary lymphoid structures (TLSs) density among patients who underwent immunochemotherapy, chemotherapy and upfront surgery using 123 tumor samples from stage-matched patients. Multiplex immunohistochemistry (mIHC) was employed to analyze the spatial distribution of PD-L1+CD11c+ cells and PD1+CD8+ T cells within TLSs. Cytometry by time-of-flight (CyTOF) was used to assess immune cell dynamics in paired biopsy and resection specimens from six patients who underwent immunochemotherapy. Key immune cells were validated in newly collected samples using flow cytometry, mIHC, and in vitro CAR-T cells model.
Patients who underwent neoadjuvant chemotherapy or immunochemotherapy exhibited increased TLSs compared to those who opted for upfront surgery. The TLS area-to-tumor area ratio distinguished pCR+MPR and NR patients in the immunochemotherapy group. Spatial analysis revealed variations in the distance between PD-L1+CD11c+ cells and PD1+CD8+ T cells within TLSs in the immunochemotherapy group. CyTOF analysis revealed an increase in the frequency of key immune cells (CCR7+CD127+CD69+CD4+ and CD38+CD8+ cells) following combined therapy. Treatment responders exhibited an increase in CCR7+CD4+ T cells, whereas CD38+CD8+ T cells were associated with compromised treatment effectiveness.
Immunochemotherapy and chemotherapy increase TLSs and granzyme B+ CD8+ T cells in tumors. The TLS area-to-tumor ratio distinguishes responders from non-responders, with PD-L1+ dendritic cells near CD8+PD-1+ T cells linked to efficacy, suggesting that PD-1 inhibitors disrupt harmful interactions. Post-immunochemotherapy, CD8+ T cells increase, but CD38+CD8+ T cells show reduced functionality. These findings highlight the complex immune dynamics and their implications for NSCLC treatment.
Copyright © 2024 Yang, You, Wang, Chen, Tang, Chen, Zhong, Song, Long, Xiang, Zhao and Xia.
Product Citations: 103
In Frontiers in Immunology on 27 December 2024 by Yang, C., You, J., et al.
-
FC/FACS
-
Cancer Research
-
Immunology and Microbiology
Induction of CD4 T cell memory responses following BCG vaccination in cattle.
In Frontiers in Veterinary Science on 12 December 2024 by Sterle, H. M., Putz, E. J., et al.
Mycobacterium bovis, the causative agent of bovine tuberculosis (bTB), is a zoonotic pathogen that contributes to economic losses in the cattle industry and poses a public health risk worldwide. Bacillus Calmette-Guerin, or BCG, is a live attenuated strain of M. bovis that is used for human vaccination against tuberculosis and is considered a potential vaccine candidate against bTB. However, BCG affords widely variable levels of protection against challenge and interferes with current diagnostic methods, and as such, it is not currently approved for use as a livestock or wildlife vaccine in the United States. Many efforts have been made to develop bTB vaccines that are reliable and do not interfere with diagnostic testing, but BCG continues to be the most effective option. Previous work has shown that a T helper 1 immune response is essential for protection against virulent M. bovis infection, characterized by CD4+ central and effector memory T cells. In an effort to identify an efficacious bTB intervention strategy, the study presented here used an in vitro recall response assay and concurrent evaluation of CD4+ T cell proliferation and cytokine production to characterize the surface and functional phenotypes of memory responses to BCG vaccination in cattle. Our findings enhance understanding of the bovine immune response to BCG and provide insights into the development of improved vaccines for the control of bTB.
Copyright © 2024 Sterle, Putz, Olsen and Boggiatto.
-
FC/FACS
-
Bos taurus (Bovine)
-
Immunology and Microbiology
-
Veterinary Research
In Clinical and Translational Medicine on 1 December 2024 by Cobo-Vuilleumier, N., Rodriguez-Fernandez, S., et al.
The complex aetiology of type 1 diabetes (T1D), characterised by a detrimental cross-talk between the immune system and insulin-producing beta cells, has hindered the development of effective disease-modifying therapies. The discovery that the pharmacological activation of LRH-1/NR5A2 can reverse hyperglycaemia in mouse models of T1D by attenuating the autoimmune attack coupled to beta cell survival/regeneration prompted us to investigate whether immune tolerisation could be translated to individuals with T1D by LRH-1/NR5A2 activation and improve islet survival.
Peripheral blood mononuclear cells (PBMCs) were isolated from individuals with and without T1D and derived into various immune cells, including macrophages and dendritic cells. Cell subpopulations were then treated or not with BL001, a pharmacological agonist of LRH-1/NR5A2, and processed for: (1) Cell surface marker profiling, (2) cytokine secretome profiling, (3) autologous T-cell proliferation, (4) RNAseq and (5) proteomic analysis. BL001-target gene expression levels were confirmed by quantitative PCR. Mitochondrial function was evaluated through the measurement of oxygen consumption rate using a Seahorse XF analyser. Co-cultures of PBMCs and iPSCs-derived islet organoids were performed to assess the impact of BL001 on beta cell viability.
LRH-1/NR5A2 activation induced a genetic and immunometabolic reprogramming of T1D immune cells, marked by reduced pro-inflammatory markers and cytokine secretion, along with enhanced mitohormesis in pro-inflammatory M1 macrophages and mitochondrial turnover in mature dendritic cells. These changes induced a shift from a pro-inflammatory to an anti-inflammatory/tolerogenic state, resulting in the inhibition of CD4+ and CD8+ T-cell proliferation. BL001 treatment also increased CD4+/CD25+/FoxP3+ regulatory T-cells and Th2 cells within PBMCs while decreasing CD8+ T-cell proliferation. Additionally, BL001 alleviated PBMC-induced apoptosis and maintained insulin expression in human iPSC-derived islet organoids.
These findings demonstrate the potential of LRH-1/NR5A2 activation to modulate immune responses and support beta cell viability in T1D, suggesting a new therapeutic approach.
LRH-1/NR5A2 activation in inflammatory cells of individuals with type 1 diabetes (T1D) reduces pro-inflammatory cell surface markers and cytokine release. LRH-1/NR5A2 promotes a mitohormesis-induced immuno-resistant phenotype to pro-inflammatory macrophages. Mature dendritic cells acquire a tolerogenic phenotype via LRH-1/NR5A2-stimulated mitochondria turnover. LRH-1/NR5A2 agonistic activation expands a CD4+/CD25+/FoxP3+ T-cell subpopulation. Pharmacological activation of LRH-1/NR5A2 improves the survival iPSC-islets-like organoids co-cultured with PBMCs from individuals with T1D.
© 2024 The Author(s). Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
-
Cell Biology
-
Immunology and Microbiology
AMPK activation induces RALDH+ tolerogenic dendritic cells by rewiring glucose and lipid metabolism.
In The Journal of Cell Biology on 7 October 2024 by Brombacher, E. C., Patente, T. A., et al.
Dendritic cell (DC) activation and function are underpinned by profound changes in cellular metabolism. Several studies indicate that the ability of DCs to promote tolerance is dependent on catabolic metabolism. Yet the contribution of AMP-activated kinase (AMPK), a central energy sensor promoting catabolism, to DC tolerogenicity remains unknown. Here, we show that AMPK activation renders human monocyte-derived DCs tolerogenic as evidenced by an enhanced ability to drive differentiation of regulatory T cells, a process dependent on increased RALDH activity. This is accompanied by several metabolic changes, including increased breakdown of glycerophospholipids, enhanced mitochondrial fission-dependent fatty acid oxidation, and upregulated glucose catabolism. This metabolic rewiring is functionally important as we found interference with these metabolic processes to reduce to various degrees AMPK-induced RALDH activity as well as the tolerogenic capacity of moDCs. Altogether, our findings reveal a key role for AMPK signaling in shaping DC tolerogenicity and suggest AMPK as a target to direct DC-driven tolerogenic responses in therapeutic settings.
© 2024 Brombacher et al.
-
Biochemistry and Molecular biology
-
Cell Biology
-
Immunology and Microbiology
Protocol to construct humanized mice with adult CD34+ hematopoietic stem and progenitor cells.
In STAR Protocols on 20 September 2024 by Yu, C. I., Maser, R., et al.
Humanized mice, defined as mice with human immune systems, have become an emerging model to study human hematopoiesis, infectious disease, and cancer. Here, we describe the techniques to generate humanized NSGF6 mice using adult human CD34+ hematopoietic stem and progenitor cells (HSPCs). We describe steps for constructing and monitoring the engraftment of humanized mice. We then detail procedures for tissue processing and immunophenotyping by flow cytometry to evaluate the multilineage hematopoietic differentiation. For complete details on the use and execution of this protocol, please refer to Yu et al.1.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
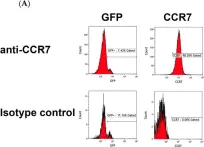
In Int J Mol Sci on 6 September 2024 by Cardona, C. I., Rodriguez, A., et al.
Fig.5.A
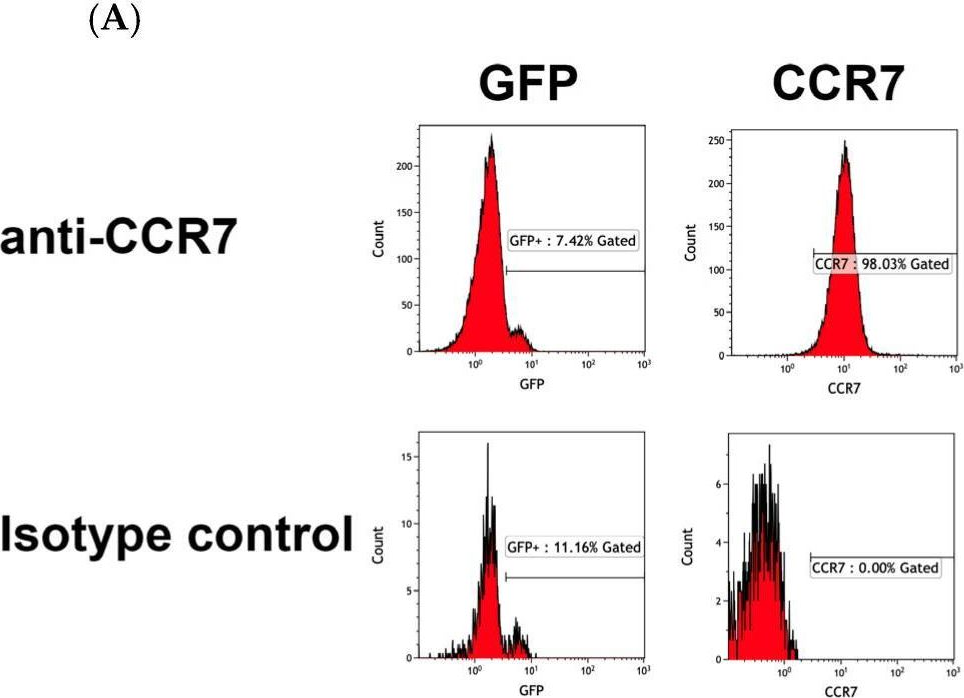
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from International Journal of Molecular Sciences by CiteAb, provided under a CC-BY license
Image 1 of 4
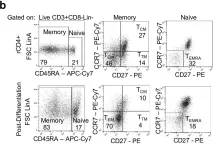
In PLoS Pathog on 1 October 2019 by Wonderlich, E. R., Subramanian, K., et al.
Fig.1.B
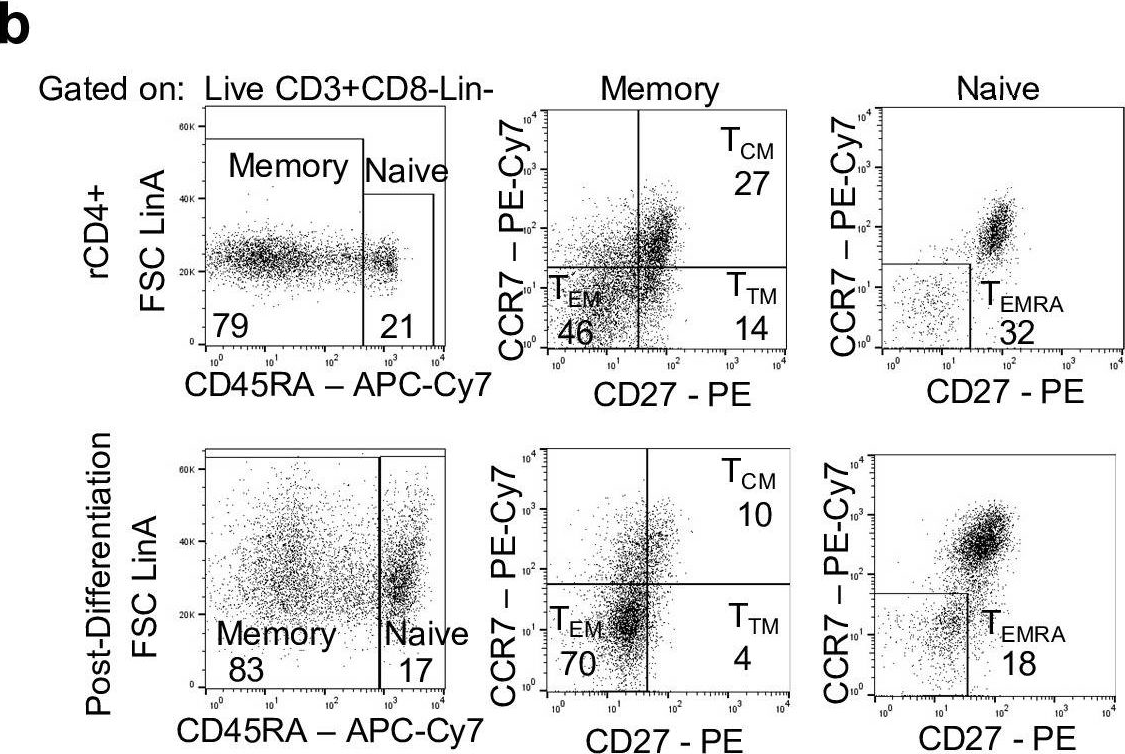
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from PLoS Pathogens by CiteAb, provided under a CC-BY license
Image 1 of 4
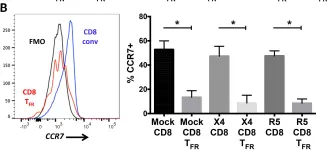
In PLoS Pathog on 1 October 2016 by Miles, B., Miller, S. M., et al.
Fig.2.B
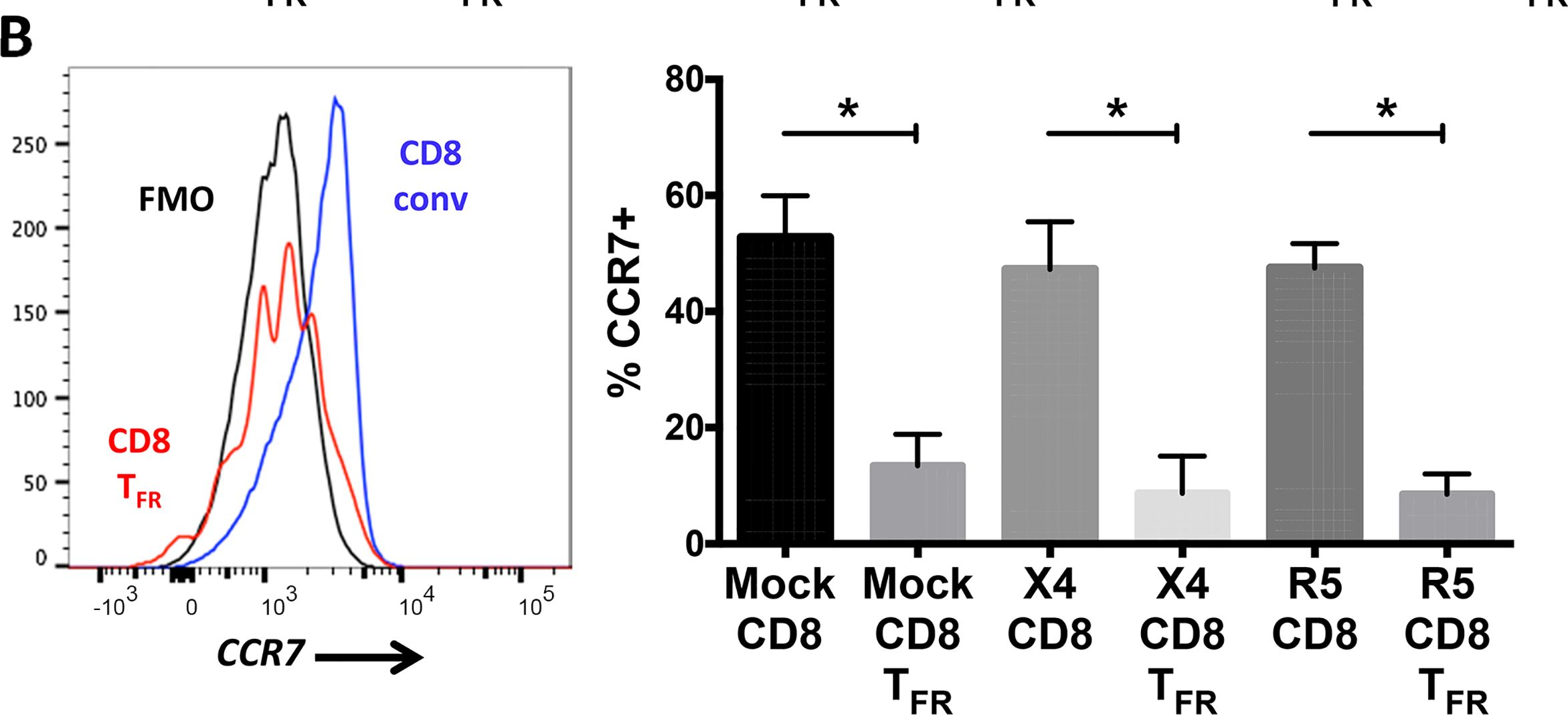
-
FC/FACS
-
Macaca mulatta (Rhesus Monkey)
Collected and cropped from PLoS Pathogens by CiteAb, provided under a CC-BY license
Image 1 of 4
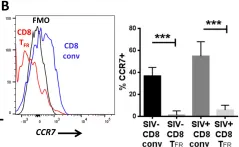
In PLoS Pathog on 1 October 2016 by Miles, B., Miller, S. M., et al.
Fig.7.B
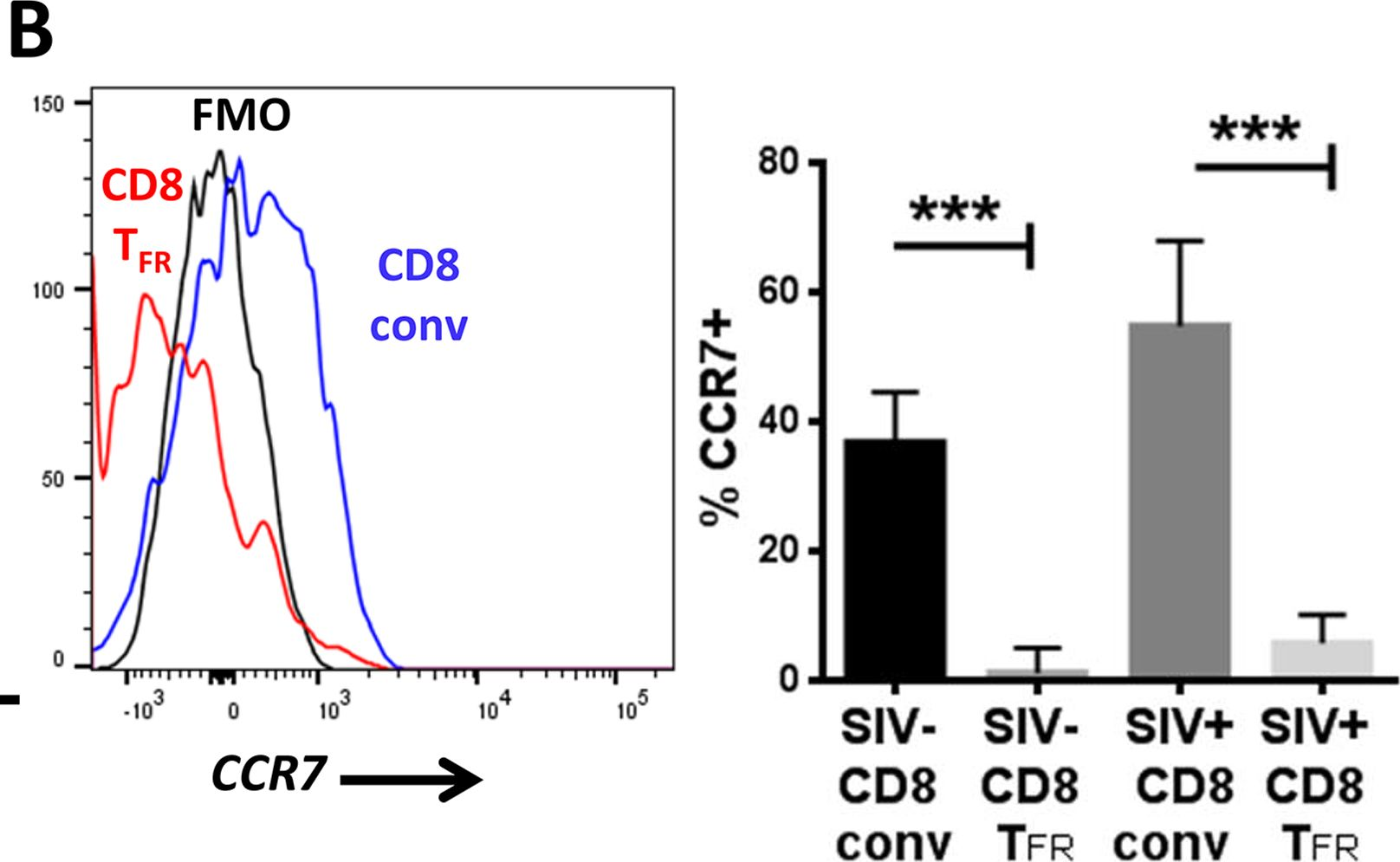
-
FC/FACS
-
Macaca mulatta (Rhesus Monkey)
Collected and cropped from PLoS Pathogens by CiteAb, provided under a CC-BY license
Image 1 of 4