The emergence of SARS-CoV-2 variants has underscored the urgent need for innovative vaccine strategies that provide robust and enduring protection against diverse strains. Our study introduces the FP-HR5 nanoparticle vaccine, designed to target the highly conserved S2 subunit of the spike protein, including the fusion peptide (FP) and heptad repeats (HR1 and HR2), using a 24-mer Helicobacter pylori ferritin platform. Administered intranasally, the FP-HR5-NP vaccine elicits robust systemic and mucosal immune responses in vivo, generating high titers of FP- and HR5-specific IgG antibodies. Notably, intranasal immunization resulted in elevated levels of secretory IgA and IgG in bronchoalveolar lavage fluid (BALF) and stimulated T-cell immune responses, significantly increasing resident memory B cells (BRM) and resident memory T cells (TRM) in the lungs. In hACE2 transgenic mice, three doses of FP-HR5-NP conferred substantial protection against Delta and Omicron variant challenges, with undetectable viral RNA levels in the lungs and no pathological changes observed. Overall, the FP-HR5-NP vaccine triggers comprehensive humoral and cellular immune responses at the mucosa, providing broad defense against SARS-CoV-2 variants and positioning it as a promising candidate for a universal COVID-19 vaccine solution.
© 2025. The Author(s).
Product Citations: 157
In Journal of Nanobiotechnology on 3 July 2025 by Liang, C., Li, R., et al.
-
COVID-19
-
Immunology and Microbiology
In Biology Methods and Protocols on 20 January 2025 by Kolobova, E. A., Petrushanko, I. Y., et al.
Alzheimer's disease (AD) is a multifactorial systemic disease that is triggered, at least in part, by the accumulation of β-amyloid (Aβ) peptides in the brain, but it also depends on immune system-mediated regulation. Recent studies suggest that B cells may play a role in AD development and point to the accumulation of clonally expanded B cells in AD patients. However, the specificity of the clonally expanded B cells is unknown, and the contribution of Aβ-specific B cells to AD pathology development is unclear. In this study, we have developed a novel method to identify Aβ-specific B cells by flow cytometry using fluorescent tetramers. The suggested method also enables the identification of B-cell clones specific to a more pathology-provoking form of Aβ with an isomerized Asp7 residue (Iso-D7-Aβ) that accumulates in elderly people and in AD patients. The method has been verified using mice immunized with antigens containing the isomerized or non-isomerized Aβ N-terminus peptides. In addition, we describe a new method for the detection of Iso-D7-Aβ-specific antibodies, which was tested on mouse serum. These methods are of potential importance in research aimed at studying AD and may be also utilized for diagnostic and therapeutic purposes.
© The Author(s) 2025. Published by Oxford University Press.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Nature Communications on 17 January 2025 by Kabrani, E., Rahjouei, A., et al.
The establishment of protective immune responses relies on the ability of terminally differentiated B cells to secrete a broad variety of antigen-specific antibodies with different effector functions. RIF1 is a multifunctional protein that promotes antibody isotype diversification via its DNA end protection activity during class switch recombination. In this study, we showed that RIF1 ablation resulted in increased plasmablast formation ex vivo and enhanced terminal differentiation into plasma cells upon immunization. Mechanistically, this phenotype is independent from RIF1's role in DNA repair and class switch recombination, and reflects its ability to modulate the transcriptional status of a subset of BLIMP1 target genes. Therefore, here we show that, in addition to promoting antibody diversification, RIF1 fine-tunes the kinetics of late B cell differentiation, thus providing an additional layer of control in the establishment of humoral immunity.
© 2025. The Author(s).
-
Mus musculus (House mouse)
-
Biochemistry and Molecular biology
-
Genetics
-
Immunology and Microbiology
In PLoS Pathogens on 1 January 2025 by Shen, Z., Li, C., et al.
Vaccines are widely regarded as one of the most effective strategies for combating infectious diseases. However, significant challenges remain, such as insufficient antibody levels, limited protection against rapidly evolving variants, and poor immune durability, particularly in subunit vaccines, likely due to their short in vivo exposure. Recent advances in extending the half-life of protein therapeutics have shown promise in improving drug efficacy, yet whether increasing in vivo persistence can enhance the efficacy of subunit vaccines remains underexplored. In this study, we developed two trimeric SARS-CoV-2 subunit vaccines with distinct pharmacokinetic profiles to evaluate the impact of vaccine persistence on immune efficacy. A self-assembling trimeric subunit vaccine (RBD-HR/trimer) was designed, followed by an extended-persistence variant (RBD-sFc-HR/trimer) incorporating a soluble monomeric IgG1 fragment crystallizable. We demonstrated that RBD-sFc-HR/trimer elicited more robust and higher levels of neutralizing antibodies, with potent and broad neutralization activity against multiple SARS-CoV-2 variants. Notably, RBD-sFc-HR/trimer induced a durable immune response, significantly increasing the number of memory B cells and T cells. This study provides critical insights for designing vaccines that achieve potent and long-lasting immune responses against infectious diseases.
Copyright: © 2025 Shen et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
Epi-microRNA mediated metabolic reprogramming counteracts hypoxia to preserve affinity maturation.
In Nature Communications on 3 December 2024 by Nakagawa, R., Llorian, M., et al.
To increase antibody affinity against pathogens, positively selected GC-B cells initiate cell division in the light zone (LZ) of germinal centers (GCs). Among these, higher-affinity clones migrate to the dark zone (DZ) and vigorously proliferate by utilizing energy provided by oxidative phosphorylation (OXPHOS). However, it remains unknown how positively selected GC-B cells adapt their metabolism for cell division in the glycolysis-dominant, cell cycle arrest-inducing, hypoxic LZ microenvironment. Here, we show that microRNA (miR)-155 mediates metabolic reprogramming during positive selection to protect high-affinity clones. Mechanistically, miR-155 regulates H3K36me2 levels in hypoxic conditions by directly repressing the histone lysine demethylase, Kdm2a, whose expression increases in response to hypoxia. The miR-155-Kdm2a interaction is crucial for enhancing OXPHOS through optimizing the expression of vital nuclear mitochondrial genes under hypoxia, thereby preventing excessive production of reactive oxygen species and subsequent apoptosis. Thus, miR-155-mediated epigenetic regulation promotes mitochondrial fitness in high-affinity GC-B cells, ensuring their expansion and consequently affinity maturation.
© 2024. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Biochemistry and Molecular biology
-
Cell Biology
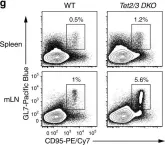
In Nat Commun on 1 May 2019 by Yue, X., Lio, C. J., et al.
Fig.4.G

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 1