Although vaccines are usually given intramuscularly, the intranasal delivery route may lead to better mucosal protection and limit the spread of respiratory virus while easing administration and improving vaccine acceptance. The challenge, however, is to achieve delivery across the selective epithelial cell barrier. Here we report on a subunit vaccine platform, in which the antigen is genetically fused to albumin to facilitate FcRn-mediated transport across the mucosal barrier in the presence of adjuvant. Intranasal delivery in conventional and transgenic mouse models induces both systemic and mucosal antigen-specific antibody responses that protect against challenge with SARS-CoV-2 or influenza A. When benchmarked against an intramuscularly administered mRNA vaccine or an intranasally administered antigen fused to an alternative carrier of similar size, only the albumin-based intranasal vaccine yields robust mucosal IgA antibody responses. Our results thus suggest that this needle-free, albumin-based vaccine platform may be suited for vaccination against respiratory pathogens.
© 2025. The Author(s).
Product Citations: 75
In Nature Communications on 1 May 2025 by Anthi, A. K., Kolderup, A., et al.
-
Immunology and Microbiology
In Haematologica on 1 April 2025 by Trifinopoulos, J., List, J., et al.
Despite major therapeutic advances in the treatment of acute lymphoblastic leukemia (ALL), resistances and long-term toxicities still pose significant challenges. Cyclins and their associated cyclin-dependent kinases are one focus of cancer research when looking for targeted therapies. We discovered cyclin C to be a key factor for B-cell ALL (B-ALL) development and maintenance. While cyclin C is not essential for normal hematopoiesis, CcncΔ/Δ BCR::ABL1+ B-ALL cells fail to elicit leukemia in mice. RNA sequencing experiments revealed a p53 pathway deregulation in CcncΔ/Δ BCR::ABL1+ cells resulting in the inability of the leukemic cells to adequately respond to stress. A genome-wide CRISPR/Cas9 loss-of-function screen supplemented with additional knock-outs unveiled a dependency of human B-lymphoid cell lines on CCNC. High cyclin C levels in B-cell precursor (BCP) ALL patients were associated with poor event-free survival and increased risk of early disease recurrence after remission. Our findings highlight cyclin C as a potential therapeutic target for B-ALL, particularly to enhance cancer cell sensitivity to stress and chemotherapy.
-
Mus musculus (House mouse)
-
Cancer Research
-
Cardiovascular biology
-
Immunology and Microbiology
In Vaccines on 3 March 2025 by Lv, Y., Tang, J., et al.
Background:Echinococcus granulosus represents a significant threat to animal husbandry and human health, but its consequences are often underestimated. Vaccination can prevent E. granulosus infection. We investigated the immune protective effect induced by the recombinant protein P29 of E. granulosus (rEg.P29) peptide vaccine. Methods: The CD4+ T-, CD8+ T-, Treg-, and CD8+CD107a+ T-cell proportions in the spleen and peripheral blood of infected mice were analyzed using flow cytometry. Additionally, we measured the proportions of IFN-γ and IL-2 secreted by memory T cells, CD19+CD138-B cells, CD19+CD138+ plasmablasts, CD19-CD138+ plasma cells, and CD19+IgD-IgG+ and CD19+IgD-IgA+ memory B cells. Results: No significant differences were noted in CD4+ T-, CD8+ T-, and CD8+CD107a+ Treg-cell percentages among the experimental groups. However, IFN-γ, IL-2, and TNF-α levels and vaccine-specific antibody concentrations in the plasma were significantly elevated in the rEg.P29T+B + CpG + infection and rEg.P29 + CpG + infection groups compared to those in the PBS + infection and CpG + infection groups. Similarly, CD19-CD138+ plasma cell and CD19+IgD-IgG+ and CD19+IgD-IgA+ memory B-cell populations, along with specific antibodies, were significantly higher in these groups. Especially, the average cyst burden in the rEg.P29T+B + CpG + infection and rEg.P29 + CpG + infection groups was significantly reduced compared to that in the PBS + infection and CpG + infection groups. Conclusions: Synthetic peptide vaccines targeting rEg.P29 can effectively inhibit cysts, offering a novel strategy for the development of vaccines against E. granulosus. These findings provide a foundation for further research on the immunogenicity and protective efficacy of rEg.P29-based vaccines.
-
Immunology and Microbiology
In Pathogens on 9 December 2024 by Vega Rojas, L. J., Ruiz-Manzano, R. A., et al.
SARS-CoV-2 (Betacoronavirus pandemicum) is responsible for the disease identified by the World Health Organization (WHO) as COVID-19. We designed "CHIVAX 2.1", a multi-epitope vaccine, containing ten immunogenic peptides with conserved B-cell and T-cell epitopes in the receceptor binding domain (RBD) sequences of different SARS-CoV-2 variants of concern (VoCs). We evaluated the immune response of mice immunized with 20 or 60 µg of the chimeric protein with two different alum adjuvants (Alhydrogel® and Adju-Phos®), plus PHAD®, in a two-immunization regimen (0 and 21 days). Serum samples were collected on days 0, 21, 31, and 72 post first immunization, with antibody titers determined by indirect ELISA, while lymphoproliferation assays and cytokine production were evaluated by flow cytometry. The presence of neutralizing antibodies was assessed by surrogate neutralization assays. Higher titers of total IgG, IgG1, and IgG2a antibodies, as well as increased proliferation rates of specific CD4+ and CD8+ T cells, were observed in mice immunized with 60 μg of protein plus Adju-Phos®/PHAD®. This formulation also generated the highest levels of TNF-α and IFN-γ, in addition to the presence of neutralizing antibodies against Delta and Omicron VoC. These findings indicate the potential of this chimeric multi-epitope vaccine with combined adjuvants as a promising platform against viral infections, eliciting a TH1 or TH1:TH2 balanced cell response.
-
Mus musculus (House mouse)
-
COVID-19
-
Immunology and Microbiology
Tumor stage-driven disruption of NK cell maturation in human and murine tumors.
In IScience on 15 November 2024 by Russick, J., Torset, C., et al.
Natural killer (NK) cells play a pivotal role against cancer, both by direct killing of malignant cells and by promoting adaptive immune response though cytokine and chemokine secretion. In the lung tumor microenvironment (TME), NK cells are scarce and dysfunctional. By conducting single-cell transcriptomic analysis of lung tumors, and exploring pseudotime, we uncovered that the intratumoral maturation trajectory of NK cells is disrupted in a tumor stage-dependent manner, ultimately resulting in the selective exclusion of the cytotoxic subset. Using functional assays, we observed intratumoral NK cell death and a reduction in cytotoxic capacities depending on the tumor stage. Finally, our analyses of human public dataset on lung cancer corroborate these findings, revealing a parallel dysfunctional maturation process of NK cells during tumor progression. These results highlight additional mechanisms by which tumor cells escape from NK cell cytotoxicity, therefore paving the way for tailored therapeutic strategies.
© 2024 The Author(s).
-
Cancer Research
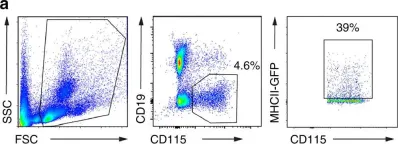
In Nat Commun on 21 February 2018 by Westhorpe, C. L. V., Norman, M. U., et al.
Fig.7.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 1