Mesenchymal stem/stromal cells (MSCs) are integral components of the tumor microenvironment and critical for the colonization of disseminated cancer cells; specifically, stem cell antigen (Sca-1) is recognized as a surface marker of MSCs. In this study, we found that MSCs highly expressing Sca-1 are positively associated with lung metastasis. MSCs derived from the lungs of mice bearing metastasized breast tumors (LMSCs) exhibited higher level of Sca-1 compared to those with adenoma. When co-injected with 4T1 cells intravenously, Sca-1high LMSCs resulted in more tumor nodules in lung tissue than Sca-1low LMSCs. Furthermore, Sca-1high LMSCs expressed higher levels of CCL2, CCL7, and CXCL1 than Sca-1low LMSCs. Sca-1high LMSCs can directly recruit 4T1 cells through producing CXCL1. Additionally, Sca-1high LMSCs are highly potent in recruiting immune cells of the myeloid lineage (neutrophils and macrophages) to the lungs. Inhibition of macrophage chemotaxis by Bindarit, an inhibitor of CCL2/7/8 transcription, decreased the lung tumor burden induced by Sca-1high MSCs. Using Ccr5-/- mice, it was further confirmed that Sca-1high LMSCs promote tumorigenesis by recruiting macrophages, further supporting that the increased recruitment of macrophages mediates the pro-metastasis effect of Sca-1high LMSCs. Collectively, this study demonstrated that Sca-1high LMSCs and their effectors could be targeted to inhibit breast cancer metastasis to the lung.
© 2025. The Author(s).
Product Citations: 245
In Cell Death & Disease on 9 July 2025 by Cao, L., Li, Y., et al.
In Nature Communications on 3 July 2025 by Fan, Z., Liu, Y., et al.
Achieving a cure is an urgent need for patients with advanced solid tumors. Here, we discover that oncolytic virus (OV) infection enhances IL-18 receptor expression but fails to increase IL-18 ligand expression. Therefore, we engineer armed oncolytic alphavirus M1 expressing wild-type IL-18 (wtIL-18) or a mutant variant (mutIL-18) that evades IL-18 binding protein (IL-18BP) while maintaining IL-18 receptor (IL-18R) binding. Intravenous administration of M1-mutIL-18 suppresses the growth of multiple advanced solid tumors in C57BL/6 and BALB/c mouse models and promotes long-term systemic immune memory. Mechanistically, armed M1-mutIL-18 enhances directed clonal expansion and differentiation of CD8+ T cells and sustains IFN-γ production. Thus, armed M1-mutIL-18 promotes dendritic cell (DC) activation, priming and activation of CD8+ T cells in lymphatic organs, and infiltration of IL-18R+ CD8+ T cells in the tumor microenvironment, establishing a positive feedback loop. We further show that a PD-L1 inhibitor enhances the anti-tumor efficacy of mutIL-18 OVs. These results highlight the importance of the IL-18 pathway in oncolytic virus therapy and implicate reprogramming ligand-receptor interaction as an effective strategy for immunotherapy.
© 2025. The Author(s).
In Nature Communications on 16 April 2025 by Schmidt, A., Fuchs, J., et al.
Lung tissue-resident memory T cells (TRM) are critical for the local control of respiratory tract infections caused by influenza A viruses (IAV). Here we compare TRM populations induced by intranasal adenoviral vector vaccines encoding hemagglutinin and nucleoprotein (NP) with those induced by an H1N1 infection in BALB/c mice. While vaccine-induced TRM express high levels of CD103 and persist longer in the lung parenchyma, short-lived, H1N1-induced TRM have a transcriptome associated with higher cytotoxic potential and distinct transcriptional profile as shown by single-cell RNA sequencing. In both the vaccine and H1N1 groups, NP-specific CD8+ T cells expand during heterologous influenza virus infection and protect the mice from disease. Meanwhile, lung inflammation in response to an infection with unrelated respiratory syncytial virus do not influence the fate of pre-existing TRM. Our preclinical work thus confirms that inflammatory conditions in the tissue shape the phenotypic and functional characteristics of TRM to serve relevant informations for optimizing mucosal vaccines.
© 2025. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Haematologica on 1 April 2025 by Trifinopoulos, J., List, J., et al.
Despite major therapeutic advances in the treatment of acute lymphoblastic leukemia (ALL), resistances and long-term toxicities still pose significant challenges. Cyclins and their associated cyclin-dependent kinases are one focus of cancer research when looking for targeted therapies. We discovered cyclin C to be a key factor for B-cell ALL (B-ALL) development and maintenance. While cyclin C is not essential for normal hematopoiesis, CcncΔ/Δ BCR::ABL1+ B-ALL cells fail to elicit leukemia in mice. RNA sequencing experiments revealed a p53 pathway deregulation in CcncΔ/Δ BCR::ABL1+ cells resulting in the inability of the leukemic cells to adequately respond to stress. A genome-wide CRISPR/Cas9 loss-of-function screen supplemented with additional knock-outs unveiled a dependency of human B-lymphoid cell lines on CCNC. High cyclin C levels in B-cell precursor (BCP) ALL patients were associated with poor event-free survival and increased risk of early disease recurrence after remission. Our findings highlight cyclin C as a potential therapeutic target for B-ALL, particularly to enhance cancer cell sensitivity to stress and chemotherapy.
-
Cancer Research
-
Cardiovascular biology
-
Immunology and Microbiology
In Cellular and Molecular Life Sciences : CMLS on 30 March 2025 by Li, M., Wu, L., et al.
Macrophages play differential roles in the pathogenesis of atherosclerosis due to their different phenotypes. Although α-SMA+ macrophages have been found to present in bone marrow and atherosclerotic plaques, their role in atherosclerosis remains unclear. By performing partial carotid ligation (PCL) on monocyte/macrophage lineage-tracked mice, we observed bone marrow-derived α-SMA+ macrophages in the subendothelium and atherosclerotic plaques under disturbed flow conditions. The functional role of α-SMA+ macrophages in atherosclerotic plaque formation was examined using macrophage-specific Acta2 knockout (Acta2MKO) mice generated by crossing Acta2f/f transgenic mice with LysM-Cre mice. The size of the aortic plaques was 77.43% smaller in Acta2MKO mice than in Acta2f/f mice following adeno-associated virus-mutant PCSK9 injection and high-fat diet (HFD) feeding for 12 weeks. A significant reduction in lipid deposition, macrophage infiltration and the α-SMA+ area was observed in the aortic roots of Acta2MKO mice compared with Acta2f/f mice. Mechanistically, using Acta2-overexpressing Raw264.7 cells (Acta2hi cells) and bone marrow-derived macrophages (BMDMs) from Acta2MKO mice (Acta2MKO BMDMs), we showed that macrophage α-SMA increased the expression of the scavenger receptor SR-A, induced Ox-LDL binding and uptake, and reduced the level of the cholesterol transporter ABCA1, potentially via the AKT pathway. Together, our results indicate that bone marrow-derived α-SMA+ macrophages contribute to atherosclerotic plaque formation due to dysregulated cholesterol uptake and efflux, providing potential targets for the prevention and treatment of atherosclerosis.
© 2025. The Author(s).
-
Biochemistry and Molecular biology
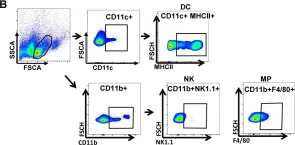
In Nutrients on 9 September 2020 by Sun, P., Kim, Y., et al.
Fig.2.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nutrients by CiteAb, provided under a CC-BY license
Image 1 of 5
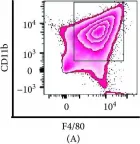
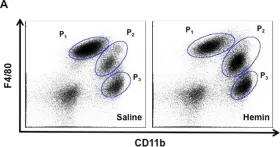
In Mediators Inflamm on 12 June 2019 by Wiktorowicz, J. E., Chowdhury, I. H., et al.
Fig.1.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Mediators Inflamm by CiteAb, provided under a CC-BY license
Image 1 of 5
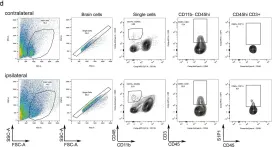
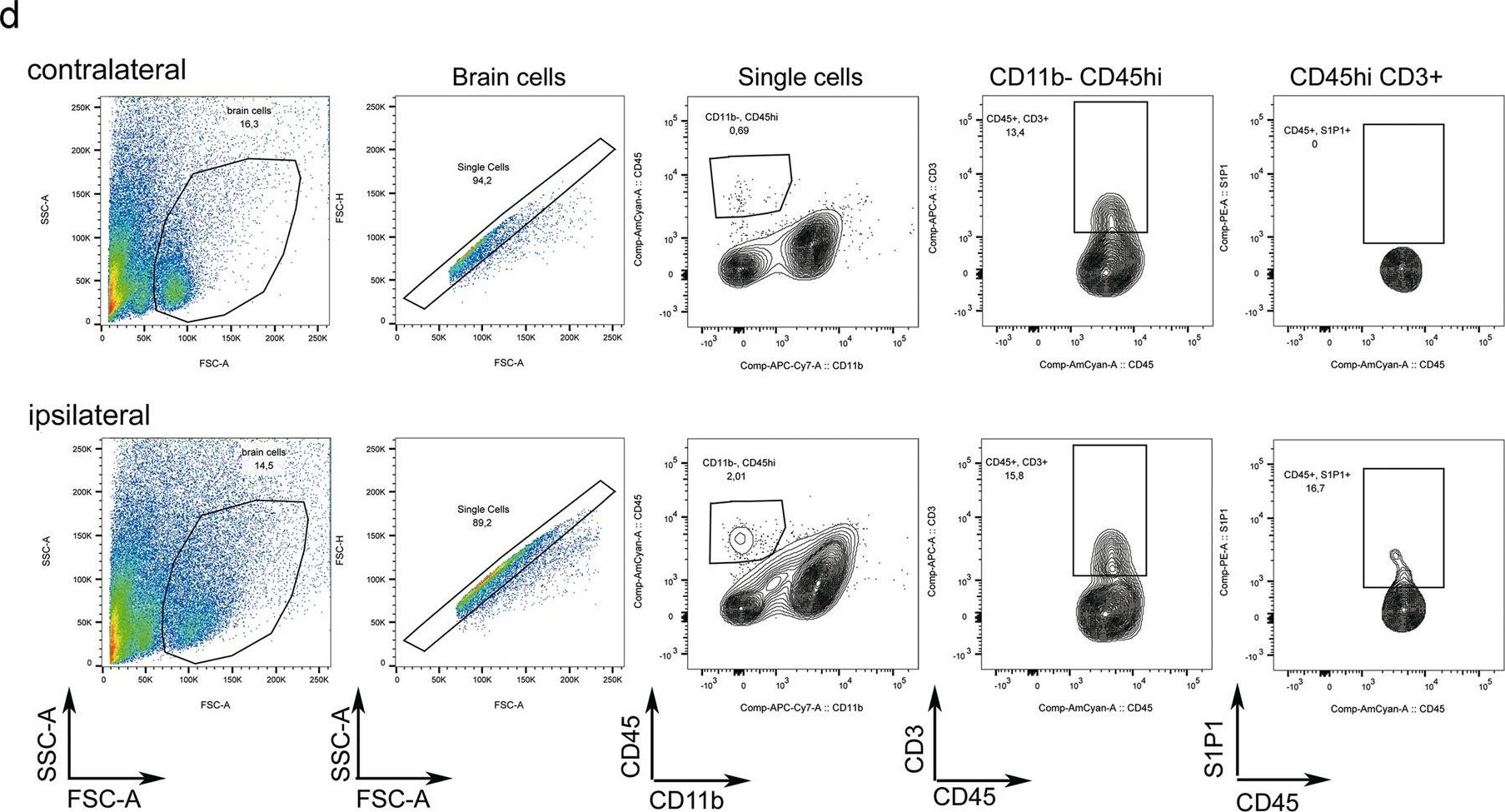
In Sci Rep on 5 June 2019 by Salas-Pérdomo, A., Miro-Mur, F., et al.
Fig.2.D
-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 5
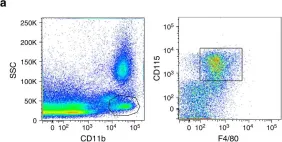
In Nat Commun on 21 February 2018 by Westhorpe, C. L. V., Norman, M. U., et al.
Fig.6.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 5
In Sci Rep on 15 March 2017 by Rossi, M., Thierry, A., et al.
Fig.8.A

-
FC/FACS
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 5