Osteoporosis is a chronic metabolic bone disease that causes excessive osteoclast formation, ultimately resulting in massive bone loss. Yougui pills (YGPs) are the classic traditional Chinese medicine formula with an anti-osteoporosis effect, but the underlying mechanisms are unknown.
The ameliorative impact of YGPs on bone loss in ovarectomized mice model was examined by Alcian Blue Haematoxylin (ABH) staining and micro-CT scanning. The "YGPs-osteoporosis-target" network was established and analyzed to explore the molecular mechanism of YGPs on osteoporosis. In addition, flow cytometry and immunohistochemistry (IHC) were used to detect the Th17 response and the expression levels of CD4, IL-17A, RANKL, and RORγt. Finally, we examined the expressions of p-P65, nuclear factor of activated T cells 1 (NFATc1), and cathepsin K (CTSK) by IHC and immunofluorescence (IF) staining to investigate the inhibitory effects of YGPs on osteoclast formation and function in vitro and in vivo.
The results of ABH staining and micro-CT scanning showed that YGPs can significantly prevent bone loss. The 'YGPs-osteoporosis-target' network found that IL-17 is the key target, and its effects may be closely related to the upstream Th17 cells and the downstream NF-κB pathway of IL-17. Meanwhile, Th17 response and the expression levels of CD4, IL-17A, RANKL, and RORγt increased in the OVX group compared with the sham group, while YGPs treatment significantly reduced the above conditions. We also found that the number and function of osteoclasts (OCs) were significantly increased by incubation with IL-17, while YGPs treatment inhibited osteoclast formation and function. Furthermore, YGPs can suppress the expression of p-P65, NFATc1, and CTSK by IHC and IF staining in vitro and in vivo.
YGPs can exert anti-osteoporosis effects by regulating osteoimmunity, and the mechanism is to inhibit osteoclast formation and bone loss by suppressing the Th17 response and IL-17/NF-κB pathway.
Product Citations: 347
In Annals of Medicine on 1 December 2025 by Zhang, P., Lin, L., et al.
In Cell Death Discovery on 5 August 2025 by Shen, L., Chen, J., et al.
Neonatal necrotizing enterocolitis (NEC) is a severe gut disease primarily affecting preterm infants, driven significantly by inflammatory macrophages. This study combined bioinformatics (single-cell/tissue RNA sequencing) and experiments to identify key macrophage changes in NEC. Analysis revealed substantial macrophage infiltration in NEC tissues. These macrophages were highly inflammatory and strongly linked to cell death pathways (ferroptosis, pyroptosis, apoptosis), with scores significantly higher than controls and correlating with inflammation. In vitro, LPS-stimulated inflammatory macrophages showed elevated ferroptosis, evidenced by cell rupture, death, increased ACSL4, decreased GPX4, iron overload, lipid peroxidation, and heightened cytokine release. Critically, the ferroptosis inhibitor Ferrostatin-1 (Fer-1) reversed these effects. While LPS alone didn't kill intestinal epithelial cells, supernatant from LPS-stimulated macrophages significantly increased intestinal epithelial cell death. Fer-1 inhibition of macrophage ferroptosis prevented this epithelial damage. In vivo, a mouse NEC model (induced by hypersomolar feeding, hypoxia, cold) displayed macrophage infiltration, inflammation, and elevated ferroptosis markers. Intraperitoneal Fer-1 administration improved intestinal injury in NEC mice. This study demonstrates that macrophage ferroptosis is a critical driver of NEC inflammation and tissue damage. Inhibiting ferroptosis with Fer-1 effectively reduces both macrophage death and subsequent intestinal epithelial injury, mitigating NEC progression. These findings highlight macrophage ferroptosis as a key therapeutic target for NEC, offering a foundation for new treatment strategies.
© 2025. The Author(s).
-
Immunology and Microbiology
In British Journal of Pharmacology on 1 August 2025 by Xuan, Y., Gao, X., et al.
Myocarditis is a life-threatening inflammatory disease, but lacks effective treatment options. Hydroxychloroquine (HCQ), an established antimalarial agent, is used widely to manage rheumatic disorders. This research aimed to evaluate the efficacy of HCQ in treating myocarditis.
A mouse model of experimental autoimmune myocarditis (EAM) was used to evaluate the therapeutic effects of HCQ on cardiac function, inflammation and fibrosis. Echocardiography, histology and cytokine assays were performed to assess cardiac function and inflammatory responses. Single-cell RNA sequencing was employed to analyse immune cell populations and chemotactic activity. C-X-C motif chemokine ligand 16 (CXCL16) levels were measured in cardiac tissue and serum, while YY1 expression was measured by western blotting in macrophages and cardiac tissue. Flow cytometry was used to evaluate immune cell infiltration and migration.
HCQ improved cardiac function in acute and chronic myocarditis. HCQ treatment reduced inflammation, fibrosis and immune cell infiltration in myocarditis models. Single-cell RNA sequencing revealed that HCQ lowered inflammatory cell proportions and suppressed macrophage chemotaxis. HCQ reduced YY1 levels, leading to the down-regulation of CXCL16 expression in macrophages and inhibition of CXCL16-mediated chemotaxis to Th17 and natural killer T (NKT) cells. CXCL16 neutralizing antibodies improved cardiac function and reduced inflammation in myocarditis.
HCQ improves cardiac function and reduces inflammation in myocarditis by inhibiting CXCL16 expression in macrophages, by suppressing its transcription factor YY1, which in turn reduced the chemotaxis of Th17 and NKT cells. HCQ is a promising therapeutic agent for myocarditis.
© 2025 British Pharmacological Society.
-
Immunology and Microbiology
-
Pharmacology
In Journal for Immunotherapy of Cancer on 31 July 2025 by Song, L., Qi, G., et al.
Immunocytokines targeting immune checkpoints have shown great potential in overcoming resistance to immune checkpoint blockade (ICB), making them a major focus of development in recent years. However, severe dose-limiting toxicity hindered their clinical application. Therefore, it is vital to develop versatile strategies to improve safety and elucidate the underlying mechanisms of resistance reversal for advancing immunocytokine therapy.
A general prodrug platform was established to construct interleukin (IL)-15 and IL-12-based immunocytokine prodrugs (P-T, P-Y, and P-Y-IL12). The efficacy of the masking strategy was validated by in vitro activity assays and in vivo safety evaluations. Antitumor efficacy of P-T was assessed in two murine cold tumor models. A comprehensive immune correlate analysis was conducted in the tumor and tumor-draining lymph node (TDLN) to identify key effector cells responsible for overcoming resistance, followed by further confirmation with egression of T cell blockade, surgical excision of TDLN, and adoptive transfer experiments. Finally, the synergistic antitumor effects of P-T with other ICB or HPK1 inhibitors were investigated.
P-T or P-Y using steric hindrance from antibody moiety Fab and Fc shields IL-15 activity in circulation, and reactivates it on cleavage by tumor-specific proteases. The universality of this masking strategy is also applicable to IL-12. Compared with prior constructs, P-T and P-Y exhibit prolonged half-life and tumor retention, facilitating sustained intratumoral immune response. P-T demonstrates reduced systemic toxicity but better control of established tumors over the unmasked counterpart. CD44+ CD8+ T cells in TDLNs are identified as critical mediators of P-T's efficacy: blockade of CD44+ CD8+ T cell trafficking into the tumor microenvironment (TME) markedly diminishes its antitumor effects. On P-T treatment, CD44+ CD8+ T cells exhibit enhanced proliferation in TDLNs and improved antitumor activity. Furthermore, P-T combined with other immunotherapies enhances antitumor effects by increasing CD44+ CD8+ T cells in TDLNs or promoting their infiltration into the TME.
The Fab and Fc-masked prodrug serves as a universal strategy for next-generation immunocytokines design, effectively addressing their dose-limiting toxicity. Additionally, leveraging immunocytokines to mobilize T cells in TDLNs offers a promising therapy option to overcome resistance to ICB and HPK1 inhibitors.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
-
Cancer Research
-
Immunology and Microbiology
Gut microbiota and SCFAs improve the treatment efficacy of chemotherapy and immunotherapy in NSCLC.
In NPJ Biofilms and Microbiomes on 28 July 2025 by Yang, Y., Ye, M., et al.
The role of gut dysbiosis in shaping immunotherapy responses is well-recognized, yet its effect on the therapeutic efficacy of chemotherapy and immunotherapy combinations remains poorly understood. We analyzed gut microbiota in non-small cell lung cancer (NSCLC) patients treated with chemo-immunotherapy, comparing responders and non-responders using 16S rRNA sequencing. Responders showed higher microbial richness and abundance of specific genera like Faecalibacterium and Subdoligranulum, and the phylum Firmicutes. Support vector machine (SVM), a machine learning model based on microbial composition, predicted treatment efficacy with the area under the curve (AUC) values of 0.763 for genera and 0.855 for species. Metagenomic analysis revealed significant differences in metabolic pathways, with responders exhibiting higher short-chain fatty acids (SCFAs) production. Fecal microbiota transplantation (FMT) and SCFAs supplementation in mouse models enhanced treatment efficacy by promoting effector T cell activity in tumors. Our study suggests that gut microbiota, through SCFAs production, regulates chemo-immunotherapy efficacy, offering new strategies to improve NSCLC treatment outcomes.
© 2025. The Author(s).
-
Immunology and Microbiology
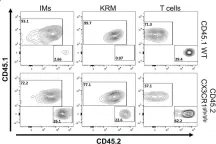
In Front Immunol on 31 May 2023 by Yashchenko, A., Bland, S. J., et al.
Fig.4.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 2
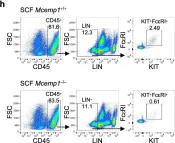
In Nat Commun on 11 April 2023 by Choi, Y. J., Yoo, J. S., et al.
Fig.2.H

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 2