Neuropathic pain, a debilitating chronic pain condition, is a major clinical challenge. The pleiotropic cytokine interleukin-4 (IL-4) has been shown to suppress neuropathic pain in rodent models, but its underlying mechanism remains unclear. Here, we show that intrathecal administration of IL-4 to mice with spinal nerve transection (SpNT) increased the number of CD11c + microglia (a microglia subset important for pain remission) in the spinal dorsal horn (SDH) and that this effect of IL-4 was essential for its ameliorating effect on SpNT-induced pain hypersensitivity. Furthermore, in mice with spared nerve injury (SNI), another model in which pain remission does not occur, the emergence of CD11c + SDH microglia was curtailed, but intrathecal IL-4 increased their emergence and ameliorated pain hypersensitivity in a CD11c + microglia-dependent manner. Our study reveals a mechanism by which intrathecal IL-4 ameliorates pain hypersensitivity after nerve injury and provides evidence that IL-4 increases CD11c + microglia with a function that ameliorates neuropathic pain.
Product Citations: 43
Interleukin-4 induces CD11c+microglia leading to amelioration of neuropathic pain in mice
Preprint on BioRxiv : the Preprint Server for Biology on 9 December 2024 by Kohno, K., Shirasaka, R., et al.
-
Mus musculus (House mouse)
-
Neuroscience
The GPCR adaptor protein Norbin controls the trafficking of C5aR1 and CXCR4 in mouse neutrophils.
In The Journal of Biological Chemistry on 1 December 2024 by Chetwynd, S. A., Ward, R. J., et al.
Norbin (Neurochondrin, NCDN) is a G protein-coupled receptor (GPCR) adaptor protein known for its importance in neuronal function. Norbin works by binding to numerous GPCRs, controlling their steady-state trafficking and sometimes their agonist-induced internalization, as well as their signaling. We recently showed that Norbin is expressed in neutrophils, limits the surface levels of the GPCRs C5aR1 and CXCR4 in neutrophils, and suppresses neutrophil-mediated innate immunity. Here, we identify C5aR1 and CXCR4 as direct Norbin interactors and used mice with myeloid-Norbin deficiency to investigate the role of Norbin in the trafficking of endogenous C5aR1 and CXCR4 in primary neutrophils by flow cytometry and cell fractionation. We show that Norbin mediates the agonist-induced internalization of C5aR1 through a β-arrestin-dependent mechanism and limits the recycling of internalized C5aR1 and CXCR4 back to the cell surface. Norbin does not control the constitutive internalization of C5aR1 and CXCR4 nor does it affect the agonist-induced internalization of CXCR4. Norbin suppresses C5aR1 signaling in mouse neutrophils by limiting the C5a-stimulated membrane translocation of Tiam1, Vav, and PKCδ, and activation of Erk and p38 Mapk pathways, as well as Gαi-dependent reactive oxygen species production. Our study demonstrates how Norbin suppresses C5aR1 and CXCR4 function in neutrophils and increases our understanding of the mechanisms through which Norbin regulates GPCR trafficking generally, by identifying its importance in β-arrestin recruitment, β-arrestin dependent agonist-induced receptor internalization, and receptor recycling.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
-
Biochemistry and Molecular biology
In EMBO Reports on 1 August 2024 by Monticone, G., Huang, Z., et al.
The COVID-19 pandemic reminded us of the urgent need for new antivirals to control emerging infectious diseases and potential future pandemics. Immunotherapy has revolutionized oncology and could complement the use of antivirals, but its application to infectious diseases remains largely unexplored. Nucleoside analogs are a class of agents widely used as antiviral and anti-neoplastic drugs. Their antiviral activity is generally based on interference with viral nucleic acid replication or transcription. Based on our previous work and computer modeling, we hypothesize that antiviral adenosine analogs, like remdesivir, have previously unrecognized immunomodulatory properties which contribute to their therapeutic activity. In the case of remdesivir, we here show that these properties are due to its metabolite, GS-441524, acting as an Adenosine A2A Receptor antagonist. Our findings support a new rationale for the design of next-generation antiviral agents with dual - immunomodulatory and intrinsic - antiviral properties. These compounds could represent game-changing therapies to control emerging viral diseases and future pandemics.
© 2024. The Author(s).
-
COVID-19
-
Genetics
The Rac-GEF Tiam1 controls integrin-dependent neutrophil responses.
In Frontiers in Immunology on 11 December 2023 by Hornigold, K., Baker, M. J., et al.
Rac GTPases are required for neutrophil adhesion and migration, and for the neutrophil effector responses that kill pathogens. These Rac-dependent functions are impaired when neutrophils lack the activators of Rac, Rac-GEFs from the Prex, Vav, and Dock families. In this study, we demonstrate that Tiam1 is also expressed in neutrophils, governing focal complexes, actin cytoskeletal dynamics, polarisation, and migration, in a manner depending on the integrin ligand to which the cells adhere. Tiam1 is dispensable for the generation of reactive oxygen species but mediates degranulation and NETs release in adherent neutrophils, as well as the killing of bacteria. In vivo, Tiam1 is required for neutrophil recruitment during aseptic peritonitis and for the clearance of Streptococcus pneumoniae during pulmonary infection. However, Tiam1 functions differently to other Rac-GEFs. Instead of promoting neutrophil adhesion to ICAM1 and stimulating β2 integrin activity as could be expected, Tiam1 restricts these processes. In accordance with these paradoxical inhibitory roles, Tiam1 limits the fMLP-stimulated activation of Rac1 and Rac2 in adherent neutrophils, rather than activating Rac as expected. Tiam1 promotes the expression of several regulators of small GTPases and cytoskeletal dynamics, including αPix, Psd4, Rasa3, and Tiam2. It also controls the association of Rasa3, and potentially αPix, Git2, Psd4, and 14-3-3ζ/δ, with Rac. We propose these latter roles of Tiam1 underlie its effects on Rac and β2 integrin activity and on cell responses. Hence, Tiam1 is a novel regulator of Rac-dependent neutrophil responses that functions differently to other known neutrophil Rac-GEFs.
Copyright © 2023 Hornigold, Baker, Machin, Chetwynd, Johnsson, Pantarelli, Islam, Stammers, Crossland, Oxley, Okkenhaug, Walker, Walker, Segonds-Pichon, Fukui, Malliri and Welch.
-
Immunology and Microbiology
In Cancer Immunology, Immunotherapy : CII on 1 November 2023 by Sun, S. H., Angell, C. D., et al.
Myeloid-derived suppressor cells (MDSC) have been linked to loss of immune effector cell function through a variety of mechanisms such as the generation of reactive oxygen and nitrogen species and the production of inhibitory cytokines. Our group has shown that signaling through Bruton's tyrosine kinase (BTK) is important for MDSC function. Ibrutinib is an orally administered targeted agent that inhibits BTK activation and is currently used for the treatment of B cell malignancies. Using a syngeneic murine model of melanoma, the effect of BTK inhibition with ibrutinib on the therapeutic response to systemic PD-L1 blockade was studied. BTK was expressed by murine MDSC and their activation was inhibited by ibrutinib. Ibrutinib was not directly cytotoxic to cancer cells in vitro, but it inhibited BTK activation in MDSC and reduced expression of inducible nitric oxide synthase (NOS2) and production of nitric oxide. Ibrutinib treatments decreased the levels of circulating MDSC in vivo and increased the therapeutic efficacy of anti-PD-L1 antibody treatment. Gene expression profiling showed that ibrutinib decreased Cybb (NOX2) signaling, and increased IL-17 signaling (upregulating downstream targets Mmp9, Ptgs2, and S100a8). These results suggest that further exploration of MDSC inhibition could enhance the immunotherapy of advanced melanoma.PrécisInhibition of Bruton's tyrosine kinase, a key enzyme in myeloid cellular function, improves therapeutic response to an anti-PD-L1 antibody in an otherwise fairly resistant murine melanoma model.
© 2023. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
-
FC/FACS
-
Cancer Research
-
Immunology and Microbiology
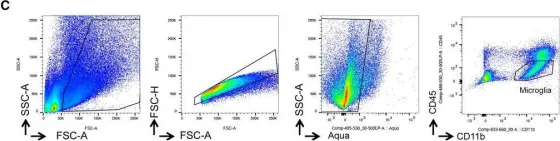
In EMBO Mol Med on 8 February 2023 by Arbaizar-Rovirosa, M., Pedragosa, J., et al.
Fig.4.C

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from EMBO Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 1