Filoviruses, including Ebola, Marburg, Sudan, and Taï Forest viruses, are zoonotic pathogens that can cause severe viral hemorrhagic fever and death. Developing vaccines that provide durable, broad immunity against multiple filoviruses is a high global health priority. In this Phase 1 trial, we enrolled 60 healthy U.S. adults and evaluated the safety, reactogenicity and immunogenicity of homologous and heterologous MVA-BN®-Filo and Ad26.ZEBOV prime-boost schedules followed in select arms by MVA-BN®-Filo boost at 1 year (NCT02891980). We found that all vaccine regimens had acceptable safety and reactogenicity. The heterologous prime-boost strategy elicited superior Ebola binding and neutralizing antibody, antibody-dependent cellular cytotoxicity (ADCC), and cellular responses compared to homologous prime-boost. The MVA-BN®-Filo boost administered at 1 year resulted in robust humoral and cellular responses that persisted through 6-month follow-up. Overall, our data demonstrated that a heterologous Ad26.ZEBOV/MVA-BN®-Filo prime-boost was safe and immunogenic and established immunologic memory primed to respond after re-exposure. Clinicaltrials.gov, NCT02891980, registered September 1, 2016.
© 2024. The Author(s).
Product Citations: 38
In NPJ Vaccines on 23 December 2024 by Rostad, C. A., Yildirim, I., et al.
-
FC/FACS
-
Homo sapiens (Human)
-
Immunology and Microbiology
In Cell Reports on 25 June 2024 by Hurtado, J., Rogers, T. F., et al.
The development of vaccines and therapeutics that are broadly effective against known and emergent coronaviruses is an urgent priority. We screened the circulating B cell repertoires of COVID-19 survivors and vaccinees to isolate over 9,000 severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-specific monoclonal antibodies (mAbs), providing an expansive view of the SARS-CoV-2-specific Ab repertoire. Among the recovered antibodies was TXG-0078, an N-terminal domain (NTD)-specific neutralizing mAb that recognizes diverse alpha- and beta-coronaviruses. TXG-0078 achieves its exceptional binding breadth while utilizing the same VH1-24 variable gene signature and heavy-chain-dominant binding pattern seen in other NTD-supersite-specific neutralizing Abs with much narrower specificity. We also report CC24.2, a pan-sarbecovirus neutralizing antibody that targets a unique receptor-binding domain (RBD) epitope and shows similar neutralization potency against all tested SARS-CoV-2 variants, including BQ.1.1 and XBB.1.5. A cocktail of TXG-0078 and CC24.2 shows protection in vivo, suggesting their potential use in variant-resistant therapeutic Ab cocktails and as templates for pan-coronavirus vaccine design.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Vaccine priming of rare HIV broadly neutralizing antibody precursors in nonhuman primates.
In Science on 17 May 2024 by Steichen, J. M., Phung, I., et al.
Germline-targeting immunogens hold promise for initiating the induction of broadly neutralizing antibodies (bnAbs) to HIV and other pathogens. However, antibody-antigen recognition is typically dominated by heavy chain complementarity determining region 3 (HCDR3) interactions, and vaccine priming of HCDR3-dominant bnAbs by germline-targeting immunogens has not been demonstrated in humans or outbred animals. In this work, immunization with N332-GT5, an HIV envelope trimer designed to target precursors of the HCDR3-dominant bnAb BG18, primed bnAb-precursor B cells in eight of eight rhesus macaques to substantial frequencies and with diverse lineages in germinal center and memory B cells. We confirmed bnAb-mimicking, HCDR3-dominant, trimer-binding interactions with cryo-electron microscopy. Our results demonstrate proof of principle for HCDR3-dominant bnAb-precursor priming in outbred animals and suggest that N332-GT5 holds promise for the induction of similar responses in humans.
-
Immunology and Microbiology
Structural conservation of Lassa virus glycoproteins and recognition by neutralizing antibodies.
In Cell Reports on 30 May 2023 by Perrett, H. R., Brouwer, P. J. M., et al.
Lassa fever is an acute hemorrhagic fever caused by the zoonotic Lassa virus (LASV). The LASV glycoprotein complex (GPC) mediates viral entry and is the sole target for neutralizing antibodies. Immunogen design is complicated by the metastable nature of recombinant GPCs and the antigenic differences among phylogenetically distinct LASV lineages. Despite the sequence diversity of the GPC, structures of most lineages are lacking. We present the development and characterization of prefusion-stabilized, trimeric GPCs of LASV lineages II, V, and VII, revealing structural conservation despite sequence diversity. High-resolution structures and biophysical characterization of the GPC in complex with GP1-A-specific antibodies suggest their neutralization mechanisms. Finally, we present the isolation and characterization of a trimer-preferring neutralizing antibody belonging to the GPC-B competition group with an epitope that spans adjacent protomers and includes the fusion peptide. Our work provides molecular detail information on LASV antigenic diversity and will guide efforts to design pan-LASV vaccines.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
-
Immunology and Microbiology
In Immunity on 14 March 2023 by Zhou, P., Song, G., et al.
Pan-betacoronavirus neutralizing antibodies may hold the key to developing broadly protective vaccines against novel pandemic coronaviruses and to more effectively respond to SARS-CoV-2 variants. The emergence of Omicron and subvariants of SARS-CoV-2 illustrates the limitations of solely targeting the receptor-binding domain (RBD) of the spike (S) protein. Here, we isolated a large panel of broadly neutralizing antibodies (bnAbs) from SARS-CoV-2 recovered-vaccinated donors, which targets a conserved S2 region in the betacoronavirus spike fusion machinery. Select bnAbs showed broad in vivo protection against all three deadly betacoronaviruses, SARS-CoV-1, SARS-CoV-2, and MERS-CoV, which have spilled over into humans in the past two decades. Structural studies of these bnAbs delineated the molecular basis for their broad reactivity and revealed common antibody features targetable by broad vaccination strategies. These bnAbs provide new insights and opportunities for antibody-based interventions and for developing pan-betacoronavirus vaccines.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
-
FC/FACS
-
Immunology and Microbiology
In Immunol Res on 1 December 2015 by Monahan, R., Stein, A., et al.
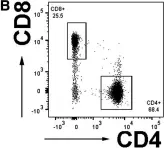
Fig.1.B

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Immunol Res by CiteAb, provided under a CC-BY license
Image 1 of 4
In Immunol Res on 1 December 2015 by Monahan, R., Stein, A., et al.
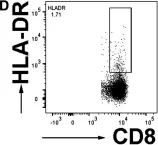
Fig.1.D

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Immunol Res by CiteAb, provided under a CC-BY license
Image 1 of 4
In Oncol Lett on 1 October 2014 by Han, L., Liu, F., et al.
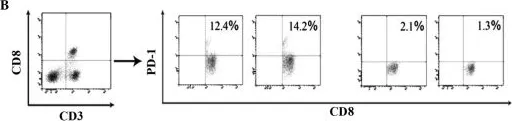
Fig.3.B

-
MACS
-
Homo sapiens (Human)
Collected and cropped from Oncol Lett by CiteAb, provided under a CC-BY license
Image 1 of 4
In Oncol Lett on 1 October 2014 by Han, L., Liu, F., et al.
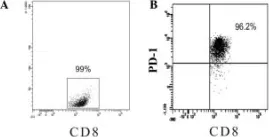
Fig.4.A

-
MACS
-
Homo sapiens (Human)
Collected and cropped from Oncol Lett by CiteAb, provided under a CC-BY license
Image 1 of 4