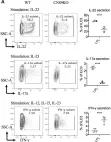
IL-17A-expressing lymphocytes, including Tc17 cells, are instrumental in immunity, immunopathology, and autoimmunity. We have previously shown that experimental attenuated live fungal vaccine-induced Tc17 cells are stable, long-lived without plasticity, and necessary to mediate sterilizing immunity during CD4+ T cell deficiency, which poses higher susceptibility to fungal infections. Cell metabolism is integral for T cell homeostasis but the metabolic adaptations of Tc17 cells are poorly defined. In this study, we hypothesized that effector Tc17 cells adopt high energy-yielding metabolic pathways to form stable, long-lived memory cells in vivo. Using a mouse model of attenuated fungal vaccination, we found that effector Tc17 cells were metabolically highly active with higher proliferation and protein synthesis than IFNγ+ CD8+ T (Tc1) cells. Glucose was necessary for effector Tc17 cell expansion but with less dependency during the late expansion despite the active metabolism. Contrary to established dogma, we found that the effector Tc17 cells preferentially channeled the glucose to OXPHOS than glycolysis, which was correlated with higher mitochondrial mass and membrane potential. Inhibition of OXPHOS shrunk the Tc17 responses while sparing Tc1 cell responses. Tc17 cells actively relied on OXPHOS throughout the expansion period, resisting adaptation to aerobic glycolysis. Our data showed that the effector Tc17 cells predominantly utilize glucose for metabolism through OXPHOS rather than aerobic glycolysis. Our study has implications in vaccine design to enhance the efficacy and immunotherapeutics to modulate the immunity and autoimmunity.
Copyright © 2025 John, Mudalagiriyappa, Chandrashekar and Nanjappa.
Product Citations: 42
Effector Tc17 cells resist shift from OXPHOS to aerobic glycolysis.
In Frontiers in Immunology on 2 June 2025 by John, R., Mudalagiriyappa, S., et al.
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Virology Journal on 5 May 2025 by Song, J. H., Son, S. E., et al.
The global spread, frequent antigenic changes, and pandemic potential of clade 2.3.4.4b highly pathogenic avian influenza H5N1 underscore the urgent need for robust cross-protective vaccines. Here, we developed a clade 2.3.4.4b H5N1 whole inactivated virus (WIV) vaccine strain with improved structural stability, productivity, and safety. By analyzing the evolutionary trends of clade 2.3.4.4b H5N1 viruses, we identified a key mutation (R90K) that increases heat stability while preserving antigenicity. Additionally, the PB2 gene of PR8 was replaced with a prototypical avian PB2 gene to increase replication efficiency in embryonated chicken eggs and reduce replication efficiency in mammalian cells, thereby improving productivity and biosafety. We found that our optimized clade 2.3.4.4b H5N1 vaccine strain (22W_KY), inactivated with binary ethylenimine (BEI), had superior antigen internalization into respiratory epithelial cells compared to those inactivated with formaldehyde or beta-propiolactone. Following intranasal administration to mice, the BEI-inactivated 22W_KY also elicited significantly stronger systemic IgG, mucosal IgA, and T-cell responses, especially in the lungs. Protective efficacy studies revealed that the BEI-inactivated 22W_KY vaccine provided complete protection against heterologous viral challenges and significant protection against heterosubtypic viral challenges, with no weight loss and complete suppression of the viral load in the respiratory tract in 2 of 3 mice. These results indicate that the BEI-inactivated 22W_KY vaccine could serve as a promising candidate for a safe, stable, cost-efficient, and broadly protective intranasal influenza vaccine against zoonotic and pandemic threats.
© 2025. The Author(s).
-
Immunology and Microbiology
The Regulation of Nucleic Acid Vaccine Responses by the Microbiome.
In The Journal of Immunology on 1 December 2023 by Johnson, A. M. F., Hager, K., et al.
Nucleic acid vaccines, including both RNA and DNA platforms, are key technologies that have considerable promise in combating both infectious disease and cancer. However, little is known about the extrinsic factors that regulate nucleic acid vaccine responses and which may determine their effectiveness. The microbiome is recognized as a significant regulator of immune development and response, whose role in regulating some traditional vaccine platforms has recently been discovered. Using germ-free and specific pathogen-free mouse models in combination with different protein, DNA, and mRNA vaccine regimens, we demonstrate that the microbiome is a significant regulator of nucleic acid vaccine immunogenicity. Although the presence of the microbiome enhances CD8+ T cell responses to mRNA lipid nanoparticle immunization, the microbiome suppresses Ig and CD4+ T cell responses to DNA-prime, DNA-protein-boost immunization, indicating contrasting roles for the microbiome in the regulation of these different nucleic acid vaccine platforms. In the case of mRNA lipid nanoparticle vaccination, germ-free mice display reduced dendritic cell/macrophage activation that may underlie the deficient vaccine response. Our study identifies the microbiome as a relevant determinant of nucleic acid vaccine response with implications for continued therapeutic development and deployment of these vaccines.
Copyright © 2023 by The American Association of Immunologists, Inc.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Immunity on 11 July 2023 by Chandra, A., Yoon, S., et al.
Multi-enhancer hubs are spatial clusters of enhancers present across numerous developmental programs. Here, we studied the functional relevance of these three-dimensional structures in T cell biology. Mathematical modeling identified a highly connected multi-enhancer hub at the Ets1 locus, comprising a noncoding regulatory element that was a hotspot for sequence variation associated with allergic disease in humans. Deletion of this regulatory element in mice revealed that the multi-enhancer connectivity was dispensable for T cell development but required for CD4+ T helper 1 (Th1) differentiation. These mice were protected from Th1-mediated colitis but exhibited overt allergic responses. Mechanistically, the multi-enhancer hub controlled the dosage of Ets1 that was required for CTCF recruitment and assembly of Th1-specific genome topology. Our findings establish a paradigm wherein multi-enhancer hubs control cellular competence to respond to an inductive cue through quantitative control of gene dosage and provide insight into how sequence variation within noncoding elements at the Ets1 locus predisposes individuals to allergic responses.
Copyright © 2023 Elsevier Inc. All rights reserved.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Biology on 13 June 2023 by Vale, D. L., Freitas, C. S., et al.
Visceral leishmaniasis (VL) in the Americas is a chronic systemic disease caused by infection with Leishmania infantum parasites. The toxicity of antileishmanial drugs, long treatment course and limited efficacy are significant concerns that hamper adequate treatment against the disease. Studies have shown the promise of an immunotherapeutics approach, combining antileishmanial drugs to reduce the parasitism and vaccine immunogens to activate the host immune system. In the current study, we developed an immunotherapy using a recombinant T cell epitope-based chimeric protein, ChimT, previously shown to be protective against Leishmania infantum, with the adjuvant monophosphoryl lipid A (MPLA) and amphotericin B (AmpB) as the antileishmanial drug. BALB/c mice were infected with L. infantum stationary promastigotes and later they received saline or were treated with AmpB, MPLA, ChimT/Amp, ChimT/MPLA or ChimT/MPLA/AmpB. The combination of ChimT/MPLA/AmpB significantly reduced the parasite load in mouse organs (p < 0.05) and induced a Th1-type immune response, which was characterized by higher ratios of anti-ChimT and anti-parasite IgG2a:IgG1 antibodies, increased IFN-γ mRNA and IFN-γ and IL-12 cytokines and accompanied by lower levels of IL-4 and IL-10 cytokines, when compared to other treatments and controls (all p < 0.05). Organ toxicity was also lower with the ChimT/MPLA/AmpB immunotherapy, suggesting that the inclusion of the vaccine and adjuvant ameliorated the toxicity of AmpB to some degree. In addition, the ChimT vaccine alone stimulated in vitro murine macrophages to significantly kill three different internalized species of Leishmania parasites and to produce Th1-type cytokines into the culture supernatants. To conclude, our data suggest that the combination of ChimT/MPLA/AmpB could be considered for further studies as an immunotherapy for L. infantum infection.
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Front Immunol on 28 March 2023 by Chang, D., Zhang, H., et al.
Fig.2.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 2
In Front Immunol on 28 March 2023 by Chang, D., Zhang, H., et al.
Fig.2.C

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 2