To study the expression of OX40 on T follicular helper (Tfh) cells and the ligand OX40L on antigen-presenting cells (APCs) in peripheral blood of patients with type 1 diabetes mellitus (T1DM) and the role of OX40 signaling in promoting Tfh cells to assist B-cell differentiation.
Cross-sectional study.
Endocrinology department of a university hospital.
Twenty-five patients with T1DM and 35 with newly diagnosed type 2 diabetes mellitus (T2DM) from January 2021 to December 2021 (39 males, 21 females; mean age: 31.0 ± 4.5, range: 19-46 years).
None.
The peripheral blood proportion of CD4+CD25-CD127+CXCR5+PD1+ Tfh cells in patients with T1DM or T2DM and the OX40L expression in CD14+ monocytes and CD19+ B cells were analyzed by flow cytometry. The OX40 signal effect on Tfh-cell function was analyzed by coincubating B cells with Tfh cells under different conditions. Flow cytometry detected the ratio of CD19-CD138+ plasmacytes.
The Tfh cells ratio and intracellular IL-21 expression in peripheral blood was significantly higher in patients with T1DM than with T2DM, and the OX40 expression in peripheral Tfh cells and OX40L expression in APC were significantly higher in T1DM. After adding OX40L protein, the CD19-CD138+-plasmacytes percentage was significantly increased and higher in T1DM. Blocking of anti-OX40L monoclonal antibodies significantly reduced the plasmacytes ratio.
The peripheral Tfh cells proportion increased and the OX40 expression in peripheral Tfh cells was upregulated in patients with T1DM vs patients with T2DM. OX40/OX40L signaling enhanced the Tfh-cell function to assist B-cell differentiation, which may contribute to the pathogenesis of T1DM.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Endocrine Society.
Product Citations: 9
In The Journal of Clinical Endocrinology and Metabolism on 15 October 2024 by Du, X., Zhu, Y., et al.
-
FC/FACS
-
Endocrinology and Physiology
-
Immunology and Microbiology
In Cancer Res Commun on 1 April 2023 by Al-Jazrawe, M., Xu, S., et al.
The interaction between neoplastic and stromal cells within a tumor mass plays an important role in cancer biology. However, it is challenging to distinguish between tumor and stromal cells in mesenchymal tumors because lineage-specific cell surface markers typically used in other cancers do not distinguish between the different cell subpopulations. Desmoid tumors consist of mesenchymal fibroblast-like cells driven by mutations stabilizing beta-catenin. Here we aimed to identify surface markers that can distinguish mutant cells from stromal cells to study tumor-stroma interactions. We analyzed colonies derived from single cells from human desmoid tumors using a high-throughput surface antigen screen, to characterize the mutant and nonmutant cells. We found that CD142 is highly expressed by the mutant cell populations and correlates with beta-catenin activity. CD142-based cell sorting isolated the mutant population from heterogeneous samples, including one where no mutation was previously detected by traditional Sanger sequencing. We then studied the secretome of mutant and nonmutant fibroblastic cells. PTX3 is one stroma-derived secreted factor that increases mutant cell proliferation via STAT6 activation. These data demonstrate a sensitive method to quantify and distinguish neoplastic from stromal cells in mesenchymal tumors. It identifies proteins secreted by nonmutant cells that regulate mutant cell proliferation that could be therapeutically.
Distinguishing between neoplastic (tumor) and non-neoplastic (stromal) cells within mesenchymal tumors is particularly challenging, because lineage-specific cell surface markers typically used in other cancers do not differentiate between the different cell subpopulations. Here, we developed a strategy combining clonal expansion with surface proteome profiling to identify markers for quantifying and isolating mutant and nonmutant cell subpopulations in desmoid tumors, and to study their interactions via soluble factors.
© 2023 The Authors; Published by the American Association for Cancer Research.
-
FC/FACS
-
Cancer Research
In Frontiers in Immunology on 27 July 2021 by Gu, C., Upchurch, K., et al.
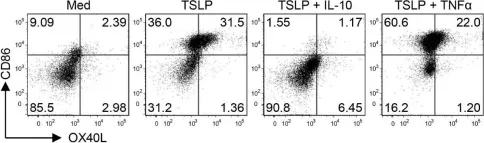
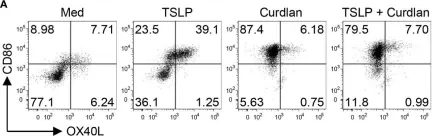
The epithelium-associated cytokine thymic stromal lymphopoietin (TSLP) can induce OX40L and CCL17 expression by myeloid dendritic cells (mDCs), which contributes to aberrant Th2-type immune responses. Herein, we report that such TSLP-induced Th2-type immune response can be effectively controlled by Dectin-1, a C-type lectin receptor expressed by mDCs. Dectin-1 stimulation induced STAT3 activation and decreased the transcriptional activity of p50-RelB, both of which resulted in reduced OX40L expression on TSLP-activated mDCs. Dectin-1 stimulation also suppressed TSLP-induced STAT6 activation, resulting in decreased expression of the Th2 chemoattractant CCL17. We further demonstrated that Dectin-1 activation was capable of suppressing ragweed allergen (Amb a 1)-specific Th2-type T cell response in allergy patients ex vivo and house dust mite allergen (Der p 1)-specific IgE response in non-human primates in vivo. Collectively, this study provides a molecular explanation of Dectin-1-mediated suppression of Th2-type inflammatory responses and suggests Dectin-1 as a target for controlling Th2-type inflammation.
Copyright © 2021 Gu, Upchurch, Horton, Wiest, Zurawski, Millard, Kane, Joo, Miller and Oh.
-
FC/FACS
-
Drosophila melanogaster (Fruit fly)
-
Homo sapiens (Human)
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In EBioMedicine on 1 May 2019 by Kristoff, J., Palma, M. L., et al.
Despite the success of antiretroviral therapy (ART), latent HIV-1 continues to persist in a long-lived population of resting memory CD4+ T cells within those who are infected. Finding a safe and effective means to induce latency reversal (LR) during ART to specifically expose this latent HIV-1 cellular reservoir for immune elimination has been a major barrier to a functional cure.
In this study, we test the use of antigen-presenting type 1-polarized, monocyte-derived dendritic cells (MDC1) generated from chronic HIV-1-infected individuals on ART as a means to induce HIV-1 latency reversal in autologous CD4+ T cells harboring replication-competent provirus. We use the same MDC1 for ex-vivo generation of autologous HIV-1 antigen-specific CD8+ cytotoxic T cells (CTL) and test their effector responses against the MDC1-exposed HIV-1- infected CD4+ T cell targets.
MDC1 presentation of either HIV-1 or cytomegalovirus (CMV) antigens to CD4+ T cells facilitated HIV-1 LR. This antigen-driven MDC1-mediated LR was sharply diminished with blockade of the CD40L/CD40 'helper' signaling pathway. Importantly, these antigen-presenting MDC1 also activated the expansion of CTL capable of killing the exposed HIV-1-infected targets.
Inclusion of virus-associated MHC class II 'helper' antigens in MDC1-based HIV-1 immunotherapies could serve both as a targeted means to safely unmask antigen-specific CD4+ T cells harboring HIV-1, and to support CTL responses that can effectively target the MDC1-exposed HIV-1 cellular reservoir as a functional cure strategy. FUND: This study was supported by the NIH-NAID grants R21-AI131763, U01-AI35041, UM1-AI126603, and T32-AI065380.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
-
FC/FACS
-
Homo sapiens (Human)
-
Immunology and Microbiology
An in vitro coculture system for the detection of sensitization following aerosol exposure.
In ALTEX on 23 February 2019 by Chary, A., Serchi, T., et al.
The aim of the study was to develop an in vitro model that mimics the alveolar-capillary barrier and that allows assessment of the respiratory sensitizing potential of respiratory sensitizers. The 3D in vitro model cultured at the air liquid interface consists of alveolar type II epithelial cells (A549), endothelial cells (EA.hy926), macrophage-like cells (PMA-differentiated THP-1) and dendritic-like cells (non-differentiated THP-1). This alveolar model was exposed apically to nebulized chemical respiratory sensitizers (Phthalic Anhydride (PA) and TriMellitic Anhydride (TMA)) or irritants (Methyl Salicylate (MeSa) and Acrolein (Acr)) at concentrations inducing at maximum 25% of cytotoxicity. The exposure to respiratory sensitizers induced dendritic cells activation and a specific cytokine release pattern, while the irritants did not. In addition, the cell surface marker OX40L was determined for dendritic like cells activation to identify high molecular weight allergens. With this in vitro model we can postulate a set of promising markers based on the studied compounds that allow the discrimination of chemical respiratory sensitizers from irritants.
In Front Immunol on 27 July 2021 by Gu, C., Upchurch, K., et al.
Fig.3.B

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 2
In Front Immunol on 27 July 2021 by Gu, C., Upchurch, K., et al.
Fig.1.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 2