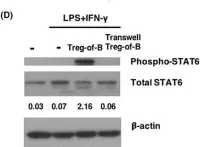
Our group have demonstrated that splenic B cells contributed to the CD4+ CD25- naive T cells conversion into CD4+ CD25+ Foxp3- regulatory T cells without adding appended cytokines, named Treg-of-B cells which were potent suppressors of adaptive immunity. We like to investigate whether Treg-of-B cells could promote alternatively activated macrophage (M2 macrophages) polarization and alleviate inflammatory disease, psoriasis. In this study, we co-cultured the bone marrow-derived macrophages (BMDMs) with Treg-of-B cells under LPS/IFN-γ stimulation and analyzed the M2-associated gene and protein using qPCR, western blotting, and immunofluorescence staining. We also examined the therapeutic effect of Treg-of-B cell-induced M2 macrophage for skin inflammation using imiquimod (IMQ)-induced psoriatic mouse model. Our results showed that BMDMs co-cultured with Treg-of-B cells upregulated typical M2-associated molecules, including Arg-1, IL-10, Pdcd1lg2, MGL-1, IL-4, YM1/2 and CD206. In an inflammatory environment, TNF-α and IL-6 production by macrophages co-cultured with Treg-of-B cells was decreased significantly. The molecular mechanism revealed that Treg-of-B cells promoted M2 macrophage polarization via STAT6 activation in a cell contact-dependent manner. Moreover, the treatment with Treg-of-B cell-induced M2 macrophages attenuated the clinical manifestations of psoriasis, such as scaling, erythema and thickening in the IMQ-induced psoriatic mouse model. T cell activation in draining lymph nodes was decreased in the Treg-of-B cell-induced M2 macrophage group after IMQ application. In conclusion, our findings suggested that Foxp3- Treg-of-B cells could induce alternatively activated M2 macrophages through STAT6 activation, providing a cell-based therapeutic strategy for psoriasis.
© 2023 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Product Citations: 11
M2-like macrophages polarized by Foxp3- Treg-of-B cells ameliorate imiquimod-induced psoriasis.
In Journal of Cellular and Molecular Medicine on 1 June 2023 by Huang, J. H., Lin, Y. L., et al.
-
WB
-
Mus musculus (House mouse)
-
Biochemistry and Molecular biology
-
Immunology and Microbiology
Static Magnetic Field Accelerates Diabetic Wound Healing by Facilitating Resolution of Inflammation.
In Journal of Diabetes Research on 31 December 2019 by Shang, W., Chen, G., et al.
Impaired wound healing is commonly encountered in patients with diabetes mellitus, which may lead to severe outcomes such as amputation, if untreated timely. Macrophage plays a critical role in the healing process including the resolution phase. Although magnetic therapy is known to improve microcirculation, its effect on wound healing remains uncertain. In the present study, we found that 0.6 T static magnetic field (SMF) significantly accelerated wound closure and elevated reepithelialization and revascularization in diabetic mice. Notably, SMF promoted the wound healing by skewing the macrophage polarization towards M2 phenotype, thus facilitating the resolution of inflammation. In addition, SMF upregulated anti-inflammatory gene expression via activating STAT6 and suppressing STAT1 in macrophage. Taken together, our results indicate that SMF may be a promising adjuvant therapeutic tool for treating diabetic wounds.
Copyright © 2019 Wenlong Shang et al.
-
WB
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Immunity on 17 December 2019 by Wang, W., Cohen, J. A., et al.
Young children are more susceptible to developing allergic asthma than adults. As neural innervation of the peripheral tissue continues to develop after birth, neurons may modulate tissue inflammation in an age-related manner. Here we showed that sympathetic nerves underwent a dopaminergic-to-adrenergic transition during post-natal development of the lung in mice and humans. Dopamine signaled through a specific dopamine receptor (DRD4) to promote T helper 2 (Th2) cell differentiation. The dopamine-DRD4 pathway acted synergistically with the cytokine IL-4 by upregulating IL-2-STAT5 signaling and reducing inhibitory histone trimethylation at Th2 gene loci. In murine models of allergen exposure, the dopamine-DRD4 pathway augmented Th2 inflammation in the lungs of young mice. However, this pathway operated marginally after sympathetic nerves became adrenergic in the adult lung. Taken together, the communication between dopaminergic nerves and CD4+ T cells provides an age-related mechanism underlying the susceptibility to allergic inflammation in the early lung.
Copyright © 2019 Elsevier Inc. All rights reserved.
-
WB
-
Mus musculus (House mouse)
-
Homo sapiens (Human)
-
Immunology and Microbiology
In Cell Reports on 25 June 2019 by Chowdhury, D., Alrefai, H., et al.
Alternatively activated (M2) macrophages promote wound healing but weaken antimicrobial defenses. The mechanisms that enforce macrophage divergence and dictate the phenotypic and metabolic characteristics of M2 macrophages remain elusive. We show that alternative activation with interleukin (IL)-4 induces expression of metallothionein 3 (MT3) that regulates macrophage polarization and function. MT3 was requisite for metabolic reprograming in IL-4-stimulated macrophages or M(IL-4) macrophages to promote mitochondrial respiration and suppress glycolysis. MT3 fostered an M(IL-4) phenotype, suppressed hypoxia inducible factor (HIF)1α activation, and thwarted the emergence of a proinflammatory M1 program in macrophages. MT3 deficiency augmented macrophage plasticity, resulting in enhanced interferon γ (IFNγ) responsiveness and a dampened M(IL-4) phenotype. Thus, MT3 programs the phenotype and metabolic fate of M(IL-4) macrophages.Published by Elsevier Inc.
-
Biochemistry and Molecular biology
-
Cell Biology
IL-4 and IL-13 Guide Early Thymic Progenitors To Mature toward Dendritic Cells.
In The Journal of Immunology on 15 November 2018 by Barik, S., Cattin-Roy, A. N., et al.
Recently we reported that IL-4 and IL-13 signaling in murine early thymic progenitors (ETPs) expressing the heteroreceptor (HR) comprising IL-4 receptor α (IL-4Rα) and IL-13 receptor α 1 (IL-13Rα1) activate STAT6 and inhibit ETP maturation potential toward T cells. In this study, we asked whether IL-4 and IL-13 signaling through the HR mobilizes other STAT molecules to shape ETP fate decision. The findings indicate that HR+ ETPs undergoing cytokine signaling display increased STAT1, but not STAT3, phosphorylation in addition to STAT6 activation. In parallel, the ETPs had a STAT1-dependent heightened expression of IRF-8, a transcription factor essential for development of CD8α+ dendritic cells (DCs). Interestingly, STAT1 phosphorylation and IRF-8 upregulation, which are independent of STAT6 activation, guided ETP maturation toward myeloid cells with a CD8α+ DC phenotype. Furthermore, these CD8α+ DCs display a thymic resident phenotype, as they did not express SIRPα, a molecule presumed to be involved in cell migration. These findings suggest that IL-4 and IL-13 cytokine-induced HR signaling provides a double-edged sword that simultaneously blocks T cell lineage potential but advances myeloid maturation that could impact T cell selection and central tolerance.
Copyright © 2018 by The American Association of Immunologists, Inc.
-
Immunology and Microbiology
In J Cell Mol Med on 1 June 2023 by Huang, J. H., Lin, Y. L., et al.
Fig.4.D

-
WB
-
Collected and cropped from J Cell Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 1