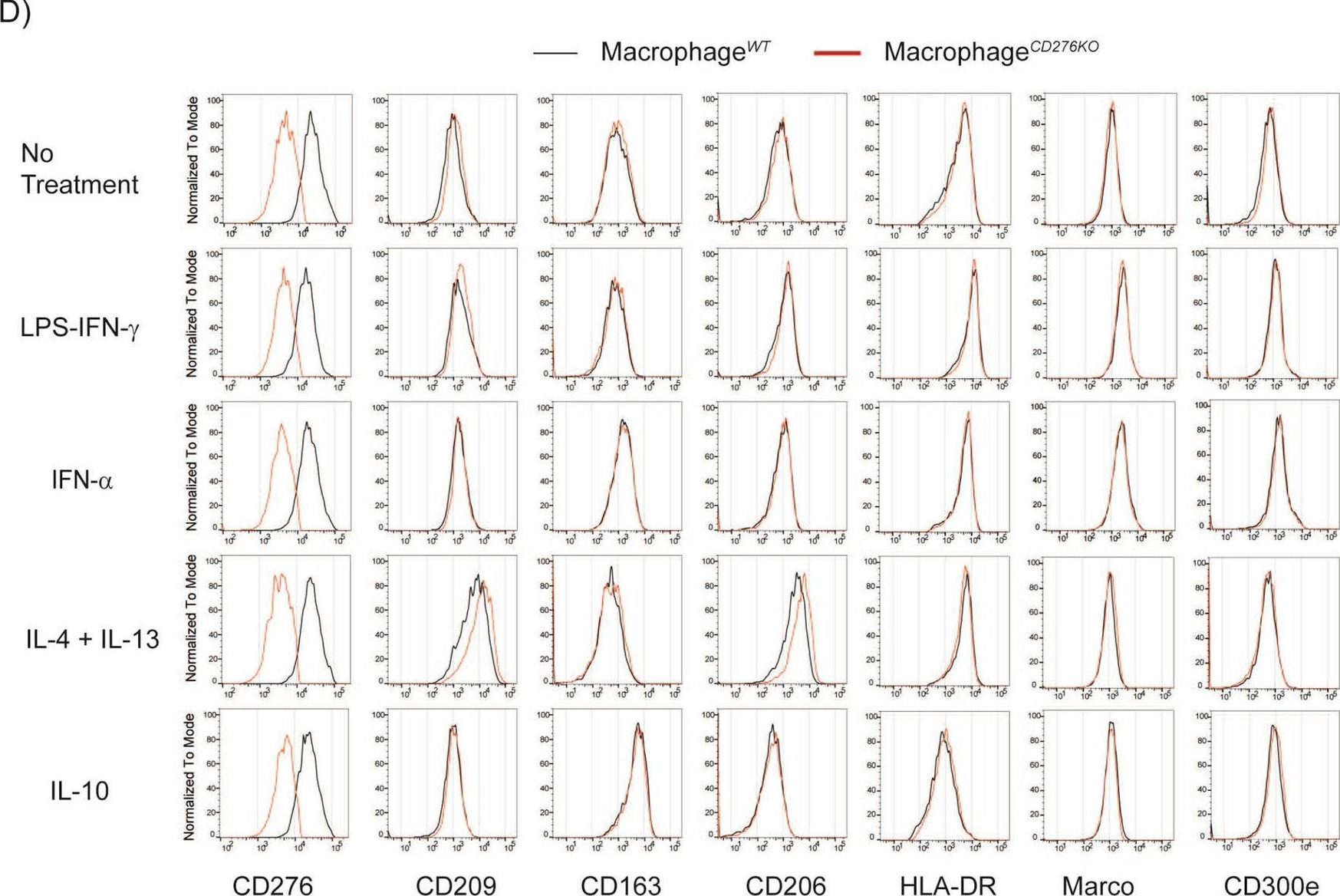
More than 70% of colorectal, prostate, ovarian, pancreatic and breast cancer specimens show expression of CD276 (B7-H3), a potential immune checkpoint family member. Several studies have shown that high CD276 expression in cancer cells correlates with a poor clinical prognosis. This has been associated with the presence of lower tumor infiltrating leukocytes. Among those, tumor-associated macrophages can comprise up to 50% of the tumor mass and are thought to support tumor growth through various mechanisms. However, a lack of information on CD276 function and interaction partner(s) impedes rigorous evaluation of CD276 as a therapeutic target in oncology. Therefore, we aimed to understand the relevance of CD276 in tumor-macrophage interaction by employing a 3D spheroid coculture system with human cells. Our data show a role for tumor-expressed CD276 on the macrophage recruitment into the tumor spheroid, and also in regulation of the extracellular matrix modulator PAI-1. Furthermore, our experiments focusing on macrophage-expressed CD276 suggest that the antibody-dependent CD276 engagement triggers predominantly inhibitory signaling networks in human macrophages.
© 2021. The Author(s).
Product Citations: 12
In Scientific Reports on 21 July 2021 by Durlanik, S., Fundel-Clemens, K., et al.
-
FC/FACS
-
Homo sapiens (Human)
-
Cancer Research
-
Immunology and Microbiology
Route of Vaccine Administration Alters Antigen Trafficking but Not Innate or Adaptive Immunity.
In Cell Reports on 24 March 2020 by Ols, S., Yang, L., et al.
Although intramuscular (i.m.) administration is the most commonly used route for licensed vaccines, subcutaneous (s.c.) delivery is being explored for several new vaccines under development. Here, we use rhesus macaques, physiologically relevant to humans, to identify the anatomical compartments and early immune processes engaged in the response to immunization via the two routes. Administration of fluorescently labeled HIV-1 envelope glycoprotein trimers displayed on liposomes enables visualization of targeted cells and tissues. Both s.c. and i.m. routes induce efficient immune cell infiltration, activation, and antigen uptake, functions that are tightly restricted to the skin and muscle, respectively. Antigen is also transported to different lymph nodes depending on route. However, these early differences do not translate into significant differences in the magnitude or quality of antigen-specific cellular and humoral responses over time. Thus, although some distinct immunological differences are noted, the choice of route may instead be motivated by clinical practicality.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
-
Immunology and Microbiology
Autologous dendritic cells and activated cytotoxic T‑cells as combination therapy for breast cancer.
In Oncology Reports on 1 February 2020 by Shevchenko, J. A., Khristin, A. A., et al.
Breast cancer is the most common oncological pathology in women worldwide. Techniques for improving the clinical parameters of patients undergoing combination therapy for breast cancer are currently under development. A type of treatment employing dendritic cells (DCs) and cytotoxic DC‑induced antigen‑specific T lymphocytes efficiently eliminates residual cancer cells that are the key cause of tumor recurrence and metastasis. In the present study, DCs and activated lymphocytes (treated with IL‑12 and IL‑18) were isolated from the peripheral blood of patients with breast cancer, using a lysate of tumor tissue as antigen. The patients received the cells as part of adjuvant or neoadjuvant regimens (stage IV disease or progression). Evaluation of immunity was performed at 3 and 6 months after terminating immunotherapy. Evaluation of the disease‑free period was performed for 3 years after surgery. The use of antigen‑loaded autologous DCs combined with mononuclear cells with increased cytotoxic activity following Th1 polarization reduced the populations of immunosuppressive cells. The results of the present study demonstrated that the investigated cellular immunotherapy for breast cancer is safe, reduces the risk of relapse and metastasis, and improves immunity by reducing the number of regulatory T cells. Therefore, this therapeutic strategy may represent a novel approach to combating distant metastases of breast cancer.
-
Homo sapiens (Human)
-
Cancer Research
-
Immunology and Microbiology
Monocytes Acquire the Ability to Prime Tissue-Resident T Cells via IL-10-Mediated TGF-β Release.
In Cell Reports on 30 July 2019 by Thompson, E. A., Darrah, P. A., et al.
Using non-human primates (NHPs), mice, and human primary cells, we found a role for interleukin-10 (IL-10) in the upregulation of the tissue-resident memory T cell (TRM) marker CD103. In NHPs, intravenous, but not subcutaneous, immunization with peptide antigen and an adjuvant combining an agonistic anti-CD40 antibody plus poly(IC:LC) induced high levels of CD103+ TRMs in the lung, which correlated with early plasma IL-10 levels. Blocking IL-10 reduced CD103 expression on human T cells stimulated in vitro with the adjuvant combination as well as diminished CD103 on lung-resident T cells in vivo in mice. Monocyte-produced IL-10 induced the release of surface-bound transforming growth factor β (TGF-β), which in turn upregulated CD103 on T cells. Early TGF-β imprinted increased sensitivity to TGF-β restimulation, indicating an early commitment of the T cell lineage toward TRMs during the priming stage of activation. IL-10-mediated TGF-β signaling may therefore have a critical role in the generation of TRM following vaccination.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
-
Immunology and Microbiology
CD161 contributes to prenatal immune suppression of IFNγ-producing PLZF+ T cells.
In The Journal of Clinical Investigation on 30 May 2019 by Halkias, J., Rackaityte, E., et al.
While the human fetal immune system defaults to a program of tolerance, there is concurrent need for protective immunity to meet the antigenic challenges encountered after birth. Activation of T cells in utero is associated with the fetal inflammatory response with broad implications for the health of the fetus and of the pregnancy. However, the characteristics of the fetal effector T cells that contribute to this process are largely unknown.
We analyzed primary human fetal lymphoid and mucosal tissues and performed phenotypic, functional, and transcriptional analysis to identify T cells with pro-inflammatory potential. The frequency and function of fetal-specific effector T cells was assessed in the cord blood of infants with localized and systemic inflammatory pathologies and compared to healthy term controls.
We identified a transcriptionally distinct population of CD4+ T cells characterized by expression of the transcription factor Promyelocytic Leukemia Zinc Finger (PLZF). PLZF+ CD4+ T cells were specifically enriched in the fetal intestine, possessed an effector memory phenotype, and rapidly produced pro-inflammatory cytokines. Engagement of the C-type lectin CD161 on these cells inhibited TCR-dependent production of IFNγ in a fetal-specific manner. IFNγ-producing PLZF+ CD4+ T cells were enriched in the cord blood of infants with gastroschisis, a natural model of chronic inflammation originating from the intestine, as well as in preterm birth, suggesting these cells contribute to fetal systemic immune activation.
Our work reveals a fetal-specific program of protective immunity whose dysregulation is associated with fetal and neonatal inflammatory pathologies.
-
FC/FACS
-
Homo sapiens (Human)
-
Immunology and Microbiology
In Sci Rep on 21 July 2021 by Durlanik, S., Fundel-Clemens, K., et al.
Fig.2.D
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 2
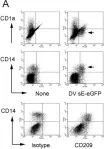
In PLoS Negl Trop Dis on 1 October 2008 by Kwan, W. H., Navarro-Sanchez, E., et al.
Fig.1.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from PLoS Negl Trop Dis by CiteAb, provided under a CC-BY license
Image 1 of 2