The proto-oncogenic epidermal growth factor receptor (EGFR) is a tyrosine kinase whose sensitivity to growth factors and signal duration determines cellular behavior. We resolve how EGFR's response to epidermal growth factor (EGF) originates from dynamically established recursive interactions with spatially organized protein tyrosine phosphatases (PTPs). Reciprocal genetic PTP perturbations enabled identification of receptor-like PTPRG/J at the plasma membrane and ER-associated PTPN2 as the major EGFR dephosphorylating activities. Imaging spatial-temporal PTP reactivity revealed that vesicular trafficking establishes a spatially distributed negative feedback with PTPN2 that determines signal duration. On the other hand, single-cell dose-response analysis uncovered a reactive oxygen species-mediated toggle switch between autocatalytically activated monomeric EGFR and the tumor suppressor PTPRG that governs EGFR's sensitivity to EGF. Vesicular recycling of monomeric EGFR unifies the interactions with these PTPs on distinct membrane systems, dynamically generating a network architecture that can sense and respond to time-varying growth factor signals.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Product Citations: 4
In Cell Systems on 26 September 2018 by Stanoev, A., Mhamane, A., et al.
In Nature Communications on 21 September 2018 by Baumdick, M., Gelléri, M., et al.
Epidermal growth factor receptor (EGFR) activation by growth factors (GFs) relies on dimerization and allosteric activation of its intrinsic kinase activity, resulting in trans-phosphorylation of tyrosines on its C-terminal tail. While structural and biochemical studies identified this EGF-induced allosteric activation, imaging collective EGFR activation in cells and molecular dynamics simulations pointed at additional catalytic EGFR activation mechanisms. To gain more insight into EGFR activation mechanisms in living cells, we develop a Förster resonance energy transfer (FRET)-based conformational EGFR indicator (CONEGI) using genetic code expansion that reports on conformational transitions in the EGFR activation loop. Comparing conformational transitions, self-association and auto-phosphorylation of CONEGI and its Y845F mutant reveals that Y845 phosphorylation induces a catalytically active conformation in EGFR monomers. This conformational transition depends on EGFR kinase activity and auto-phosphorylation on its C-terminal tail, generating a looped causality that leads to autocatalytic amplification of EGFR phosphorylation at low EGF dose.
-
Genetics
In Science Signaling on 31 July 2018 by Stallaert, W., Brüggemann, Y., et al.
The ability of cells to adapt their response to growth factors in relation to their environment is an essential aspect of tissue development and homeostasis. We found that signaling mediated by the Eph family of receptor tyrosine kinases from cell-cell contacts changed the cellular response to the growth factor EGF by modulating the vesicular trafficking of its receptor, EGFR. Eph receptor activation trapped EGFR in Rab5-positive early endosomes by inhibiting Akt-dependent vesicular recycling. By altering the spatial distribution of EGFR activity, EGF-promoted Akt signaling from the plasma membrane was suppressed, thereby inhibiting cell migration. In contrast, ERK signaling from endosomal EGFR was preserved to maintain a proliferative response to EGF stimulation. We also found that soluble extracellular signals engaging the G protein-coupled receptor Kiss1 (Kiss1R) similarly suppressed EGFR vesicular recycling to inhibit EGF-promoted migration. Eph or Kiss1R activation also suppressed EGF-promoted migration in Pten-/- mouse embryonic fibroblasts, which exhibit increased constitutive Akt activity, and in MDA-MB-231 triple-negative breast cancer cells, which overexpress EGFR. The cellular environment can thus generate context-dependent responses to EGF stimulation by modulating EGFR vesicular trafficking dynamics.Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
-
Biochemistry and Molecular biology
In eLife on 26 November 2015 by Baumdick, M., Brüggemann, Y., et al.
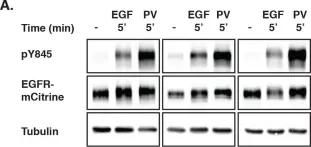
Autocatalytic activation of epidermal growth factor receptor (EGFR) coupled to dephosphorylating activity of protein tyrosine phosphatases (PTPs) ensures robust yet diverse responses to extracellular stimuli. The inevitable tradeoff of this plasticity is spontaneous receptor activation and spurious signaling. We show that a ligand-mediated switch in EGFR trafficking enables suppression of spontaneous activation while maintaining EGFR's capacity to transduce extracellular signals. Autocatalytic phosphorylation of tyrosine 845 on unliganded EGFR monomers is suppressed by vesicular recycling through perinuclear areas with high PTP1B activity. Ligand-binding results in phosphorylation of the c-Cbl docking tyrosine and ubiquitination of the receptor. This secondary signal relies on EGF-induced EGFR self-association and switches suppressive recycling to directional trafficking. The re-routing regulates EGFR signaling response by the transit-time to late endosomes where it is switched-off by high PTP1B activity. This ubiquitin-mediated switch in EGFR trafficking is a uniquely suited solution to suppress spontaneous activation while maintaining responsiveness to EGF.
-
WB
In Elife on 26 November 2015 by Baumdick, M., Brüggemann, Y., et al.
Fig.1.A

-
WB
-
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 1