Metabolic-endotoxemia, characterized by the translocation of lipopolysaccharide (LPS) from Gram-negative bacteria into the bloodstream, is a key contributor to chronic low-grade inflammation associated with obesity and type 2 diabetes. This condition exacerbates metabolic disruptions by activating Toll-like receptor 4 (TLR4) on macrophages, leading to the release of pro-inflammatory cytokines and subsequent insulin resistance. Eicosapentaenoic acid (EPA; C20:5 (n-3)), an omega-3 polyunsaturated fatty acid, has demonstrated anti-inflammatory and antioxidative properties, but its precise mechanisms of action in mitigating LPS-induced stress remain unclear. This study investigates the pathways through which C20:5 (n-3) alleviates LPS-induced oxidative stress and inflammation in macrophages. C20:5 (n-3) pretreatment significantly reduced LPS-induced inflammatory responses, decreasing IL-1β and IL-6 expression and IL-1β secretion, and lowering the percentage of HLA-DR+ macrophages. C20:5 (n-3) also attenuated ER stress, evidenced by reduced expression of ATF4, DDIT3, HSPA5/GRP78, BIP, and CHOP at both gene and protein levels. Oxidative stress was mitigated, as shown by decreased HIF1α expression, reduced ROS levels, and preservation of mitochondrial membrane potential. Importantly, C20:5 (n-3) increased the expression of PPARα and FABP5 while inhibiting NF-κB activation independently of the TLR4-IRF5 pathway. The protective effects of C20:5 (n-3) was abolished by PPARα inhibition with GW9662, indicating that C20:5 (n-3)'s action is PPARα-dependent. This study highlights the modulatory role of C20:5 (n-3) in alleviating LPS-induced oxidative stress and inflammation in macrophages through activation of the FABP5/PPARα/NF-κB axis, independently of TLR4-IRF5 signaling. These findings reveal a novel mechanism for C20:5 (n-3)'s anti-inflammatory effects and suggest that targeting the FABP5/PPARα pathway may offer therapeutic potential for treating metabolic disorders associated with chronic inflammation.
Copyright © 2025 Haya AlAbduljader et al. Oxidative Medicine and Cellular Longevity published by John Wiley & Sons Ltd.
Product Citations: 25
Eicosapentaenoic Acid (EPA) Alleviates LPS-Induced Oxidative Stress via the PPARα-NF-κB Axis.
In Oxidative Medicine and Cellular Longevity on 18 June 2025 by AlAbduljader, H., AlSaeed, H., et al.
-
ICC
-
Mus musculus (House mouse)
In Frontiers in Nutrition on 8 May 2025 by Lian, J., Men, Z., et al.
Folic acid has been associated with fetal development, especially in fetal immunity. Therefore, limited evidence regarding the effects of different folic acid supplementation of hepatitis B surface antigen (HBsAg) positive mothers in innate immunity in offspring. Herein, this study aimed to explore the association between folic acid supplementation and the innate immunity of neonates and the immunological efficacy of hepatitis B vaccine (HepB), which may provide insights that could inform pre-pregnancy health management in HBsAg-positive mothers.
It is an ambispective cohort study with 293 pairs of HBsAg-positive mothers-offspring in Taiyuan, Shanxi Province, China. Mothers were classified into three groups according to the time of starting folic acid supplementation, non-supplementation group, pre-pregnancy group and post-pregnancy supplementation group. Immunological indexes such as immune cells proportion and innate immune mediators in cord blood and anti-HBs in infants were measured. Differences in immunological indexes were analyzed by One-Way ANOVA test. Univariate and multivariate analyses were performed for factors associated with abnormal immunological indexes and potential confounders were adjusted.
The preconception folic acid group showed a significantly higher expression levels of STING (P = 0.005) and pNF-κB (P = 0.010) in cord blood along with higher anti-HBs titres (P = 0.006), when compared to both non-supplementation group and post-pregnancy supplementation group. Higher anti-HBs levels indicate a stronger immune response to HepB and may enhance protection against HBV infection during early life. Infants in the high pNF-κB expression group exhibited a significantly elevated seropositive rate of HepB compared to those in the low pNF-κB expression group (P = 0.037). There were no mediation effects and no moderation effects in this study, potentially due to the direct influence of folic acid supplementation on immune responses or the limited sample size.
In conclusion, our findings demonstrate that preconception folic acid supplementation may enhance HepB vaccine responsiveness in infants of HBsAg-positive mothers. Meanwhile, high pNF-κB expression in cord blood can increase seropositive rates in infants. This discovery has significant public health implications, as it may provide a simple and accessible intervention to improve vaccination outcomes and reduce HBV transmission in endemic regions.
Copyright © 2025 Lian, Men, Xu, Li, Li, Wang, Yao, Li, Qu, Feng and Wang.
-
Immunology and Microbiology
Eicosapentaenoic Acid (EPA) Alleviates LPS-Induced Oxidative Stress via the PPARα–NF-κB Axis
Preprint on BioRxiv : the Preprint Server for Biology on 17 March 2025 by AlAbduljader, H., AlSaeed, H., et al.
Metabolic-endotoxemia, characterized by the translocation of lipopolysaccharide (LPS) from Gram-negative bacteria into the bloodstream, is a key contributor to chronic low-grade inflammation associated with obesity and type 2 diabetes. This condition exacerbates metabolic disruptions by activating Toll-like receptor 4 (TLR4) on macrophages, leading to the release of pro-inflammatory cytokines and subsequent insulin resistance. Eicosapentaenoic acid (EPA/C20:5), an omega-3 polyunsaturated fatty acid, has demonstrated anti-inflammatory and antioxidative properties, but its precise mechanisms of action in mitigating LPS-induced stress remain unclear. This study investigates the pathways through which EPA/C20:5 alleviates LPS-induced oxidative stress and inflammation in macrophages. EPA pretreatment significantly reduced LPS-induced inflammatory responses, decreasing IL-1β and IL-6 expression and IL-1β secretion, and lowering the percentage of HLA-DR⁺ macrophages. EPA also attenuated ER stress, evidenced by reduced expression of ATF4, DDIT3, HSPA5/GRP78, BIP, and CHOP at both gene and protein levels. Oxidative stress was mitigated, as shown by decreased HIF1α expression, reduced ROS levels, and preservation of mitochondrial membrane potential. Importantly, EPA increased the expression of PPARα and FABP5 while inhibiting NF-κB activation independently of the TLR4-IRF5 pathway. The protective effects of EPA were abolished by PPARα inhibition with GW9662, indicating that EPA’s action is PPARα-dependent. This study highlights the modulatory role of EPA in alleviating LPS-induced oxidative stress and inflammation in macrophages through activation of the FABP5/PPARα/NF-κB axis, independently of TLR4-IRF5 signaling. These findings reveal a novel mechanism for EPA’s anti-inflammatory effects and suggest that targeting the FABP5/PPARα pathway may offer therapeutic potential for treating metabolic disorders associated with chronic inflammation.
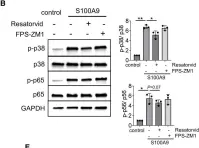
S100A9 and HMGB1 orchestrate MDSC-mediated immunosuppression in melanoma through TLR4 signaling.
In Journal for Immunotherapy of Cancer on 11 September 2024 by Ozbay Kurt, F. G., Cicortas, B. A., et al.
Immunotherapies for malignant melanoma are challenged by the resistance developed in a significant proportion of patients. Myeloid-derived suppressor cells (MDSC), with their ability to inhibit antitumor T-cell responses, are a major contributor to immunosuppression and resistance to immune checkpoint therapies in melanoma. Damage-associated molecular patterns S100A8, S100A9, and HMGB1, acting as toll like receptor 4 (TLR4) and receptor for advanced glycation endproducts (RAGE) ligands, are highly expressed in the tumor microenvironment and drive MDSC activation. However, the role of TLR4 and RAGE signaling in the acquisition of MDSC immunosuppressive properties remains to be better defined. Our study investigates how the signaling via TLR4 and RAGE as well as their ligands S100A9 and HMGB1, shape MDSC-mediated immunosuppression in melanoma.
MDSC were isolated from the peripheral blood of patients with advanced melanoma or generated in vitro from healthy donor-derived monocytes. Monocytes were treated with S100A9 or HMGB1 for 72 hours. The immunosuppressive capacity of treated monocytes was assessed in the inhibition of T-cell proliferation assay in the presence or absence of TLR4 and RAGE inhibitors. Plasma levels of S100A8/9 and HMGB1 were quantified by ELISA. Single-cell RNA sequencing (scRNA-seq) was performed on monocytes from patients with melanoma and healthy donors.
We showed that exposure to S100A9 and HMGB1 converted healthy donor-derived monocytes into MDSC through TLR4 signaling. Our scRNA-seq data revealed in patient monocytes enriched inflammatory genes, including S100 and those involved in NF-κB and TLR4 signaling, and a reduced major histocompatibility complex II gene expression. Furthermore, elevated plasma S100A8/9 levels correlated with shorter progression-free survival in patients with melanoma.
These findings highlight the critical role of TLR4 and, to a lesser extent, RAGE signaling in the conversion of monocytes into MDSC-like cells, underscore the potential of targeting S100A9 to prevent this conversion, and highlight the prognostic value of S100A8/9 as a plasma biomarker in melanoma.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
-
FC/FACS
-
WB
-
Homo sapiens (Human)
-
Cancer Research
IRAK4 degrader in hidradenitis suppurativa and atopic dermatitis: a phase 1 trial.
In Nature Medicine on 1 December 2023 by Ackerman, L., Acloque, G., et al.
Toll-like receptor-driven and interleukin-1 (IL-1) receptor-driven inflammation mediated by IL-1 receptor-associated kinase 4 (IRAK4) is involved in the pathophysiology of hidradenitis suppurativa (HS) and atopic dermatitis (AD). KT-474 (SAR444656), an IRAK4 degrader, was studied in a randomized, double-blind, placebo-controlled phase 1 trial where the primary objective was safety and tolerability. Secondary objectives included pharmacokinetics, pharmacodynamics and clinical activity in patients with moderate to severe HS and in patients with moderate to severe AD. KT-474 was administered as a single dose and then daily for 14 d in 105 healthy volunteers (HVs), followed by dosing for 28 d in an open-label cohort of 21 patients. Degradation of IRAK4 was observed in HV blood, with mean reductions after a single dose of ≥93% at 600-1,600 mg and after 14 daily doses of ≥95% at 50-200 mg. In patients, similar IRAK4 degradation was achieved in blood, and IRAK4 was normalized in skin lesions where it was overexpressed relative to HVs. Reduction of disease-relevant inflammatory biomarkers was demonstrated in the blood and skin of patients with HS and patients with AD and was associated with improvement in skin lesions and symptoms. There were no drug-related infections. These results, from what, to our knowledge, is the first published clinical trial using a heterobifunctional degrader, provide initial proof of concept for KT-474 in HS and AD to be further confirmed in larger trials. ClinicalTrials.gov identifier: NCT04772885 .
© 2023. The Author(s).
-
FC/FACS
-
Homo sapiens (Human)
In J Immunother Cancer on 11 September 2024 by Ozbay Kurt, F. G., Cicortas, B. A., et al.
Fig.2.B

-
WB
-
Homo sapiens (Human)
Collected and cropped from J Immunother Cancer by CiteAb, provided under a CC-BY license
Image 1 of 2
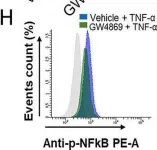
In Sci Rep on 8 October 2020 by Al-Rashed, F., Ahmad, Z., et al.
Fig.4.H

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 2