Janus kinases (JAK) mediate signaling pathways of multiple cytokines, including interferon-γ (IFN-γ), which plays a pivotal role in rheumatoid arthritis (RA) pathogenesis. Although JAK inhibitors (JAKi) have demonstrated efficacy for RA, their molecular effects on macrophages remain incompletely understood. We investigate the impact of JAKi on IFN-γ-induced gene expression in human macrophages, uncovering that JAKi selectively modulates only a subset of IFN-γ-induced genes. Integrated transcriptomic and epigenomic analyses demonstrate that JAKi effectively inhibits IFN-γ signature genes associated with IRF1-STAT1-dependent accessible chromatin regions. However, genes regulated by AP-1 and C/EBP remain insensitive to JAKi and overlap significantly with TNF-induced genes. Single-cell analysis of RA patient samples identifies macrophage subpopulations with variable JAKi sensitivity. Certain JAKi-insensitive genes in IFN-γ-primed macrophages are suppressed by JNK inhibitors. Our findings elucidate JAKi responsiveness mechanisms through IFN-γ-induced epigenomic remodeling, providing insights into inflammatory regulation in RA and suggesting strategies to overcome JAKi resistance.
© 2025 The Author(s).
Product Citations: 11
In IScience on 16 May 2025 by Kwon, G., Park, Y., et al.
Distinct Assemblies of Heterodimeric Cytokine Receptors Govern Stemness Programs in Leukemia.
In Cancer Discovery on 4 August 2023 by Kan, W. L., Dhagat, U., et al.
Leukemia stem cells (LSC) possess distinct self-renewal and arrested differentiation properties that are responsible for disease emergence, therapy failure, and recurrence in acute myeloid leukemia (AML). Despite AML displaying extensive biological and clinical heterogeneity, LSC with high interleukin-3 receptor (IL3R) levels are a constant yet puzzling feature, as this receptor lacks tyrosine kinase activity. Here, we show that the heterodimeric IL3Rα/βc receptor assembles into hexamers and dodecamers through a unique interface in the 3D structure, where high IL3Rα/βc ratios bias hexamer formation. Importantly, receptor stoichiometry is clinically relevant as it varies across the individual cells in the AML hierarchy, in which high IL3Rα/βc ratios in LSCs drive hexamer-mediated stemness programs and poor patient survival, while low ratios mediate differentiation. Our study establishes a new paradigm in which alternative cytokine receptor stoichiometries differentially regulate cell fate, a signaling mechanism that may be generalizable to other transformed cellular hierarchies and of potential therapeutic significance.
Stemness is a hallmark of many cancers and is largely responsible for disease emergence, progression, and relapse. Our finding that clinically significant stemness programs in AML are directly regulated by different stoichiometries of cytokine receptors represents a hitherto unexplained mechanism underlying cell-fate decisions in cancer stem cell hierarchies. This article is highlighted in the In This Issue feature, p. 1749.
©2023 The Authors; Published by the American Association for Cancer Research.
-
Cancer Research
In Immunity on 13 December 2022 by Lukhele, S., Rabbo, D. A., et al.
Type I and II interferons (IFNs) stimulate pro-inflammatory programs that are critical for immune activation, but also induce immune-suppressive feedback circuits that impede control of cancer growth. Here, we sought to determine how these opposing programs are differentially induced. We demonstrated that the transcription factor interferon regulatory factor 2 (IRF2) was expressed by many immune cells in the tumor in response to sustained IFN signaling. CD8+ T cell-specific deletion of IRF2 prevented acquisition of the T cell exhaustion program within the tumor and instead enabled sustained effector functions that promoted long-term tumor control and increased responsiveness to immune checkpoint and adoptive cell therapies. The long-term tumor control by IRF2-deficient CD8+ T cells required continuous integration of both IFN-I and IFN-II signals. Thus, IRF2 is a foundational feedback molecule that redirects IFN signals to suppress T cell responses and represents a potential target to enhance cancer control.
Copyright © 2022 Elsevier Inc. All rights reserved.
-
Biochemistry and Molecular biology
-
Cancer Research
-
Immunology and Microbiology
Preprint on BioRxiv : the Preprint Server for Biology on 21 June 2022 by Aron, J. L., Thauland, T., et al.
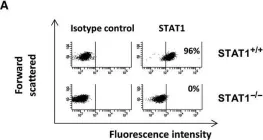
h4>Background/h4> The search for a single, pathogenic genetic variant in a patient suspected to have a monogenic inborn error of immunity (IEI) often reveals a multitude of rare variants of unknown significance (VUS). Distinguishing which VUS is disease-causing versus the irrelevant, rare variants from the genetic background is slow and difficult. Advances in gene editing technology, particularly CRISPR/Cas9, promise to accelerate the timeline for the development of single-variant animal models, thus affording an experimental system for validating new genes and their variants. h4>Objective/h4> We sought to demonstrate a proof-of-concept of using CRISPR/Cas9 in human hematopoietic stem cells (hHSC) to develop of humanized mice bearing a hematopoietic deficiency in signal transducer and activator 1 (STAT1). h4>Methods/h4> Using CRISPR/Cas9, we introduced indels into the STAT1 gene of hHSCs and implanted them into immunodeficient mice. The reconstituted immune systems were assessed by flow cytometry. h4>Results/h4> Mice transplanted with cells edited to eliminate STAT1 developed human immune systems with diverse cell phenotypes. Lymphocytes from these reconstituted mice showed low expression of STAT1 protein and diminished phosphorylation of STAT1 in response to interferon stimulation. These data mirror the impaired, but not abolished, response to interferons seen in human partial STAT1 deficiency. CRISPR/Cas9 genome editing techniques can be used to rapidly and inexpensively create functional, humanized models of primary immune deficiencies.
-
Biochemistry and Molecular biology
-
Immunology and Microbiology
IL-27 induces IFN/STAT1-dependent genes and enhances function of TIGIT+ HIVGag-specific T cells.
In IScience on 21 January 2022 by Cheng, J., Myers, T. G., et al.
HIV-specific T cells have diminished effector function and fail to control/eliminate the virus. IL-27, a member of the IL-6/IL-12 cytokine superfamily has been shown to inhibit HIV replication. However, whether or not IL-27 can enhance HIV-specific T cell function is largely unknown. In the present manuscript, we investigated the role of IL-27 signaling in human T cells by evaluating the global transcriptional changes related to the function of HIV-specific T cells. We found that T cells from people living with HIV (PLWH), expressed higher levels of STAT1 leading to enhanced STAT1 activation upon IL-27 stimulation. Observed IL-27 induced transcriptional changes were associated with IFN/STAT1-dependent pathways in CD4 and CD8 T cells. Importantly, IL-27 dependent modulation of T-bet expression promoted IFNγ secretion by TIGIT+HIVGag-specific T cells. This new immunomodulatory effect of IL-27 on HIV-specific T cell function suggests its potential therapeutic use in cure strategies.
© 2021.
-
Immunology and Microbiology
In J Cell Mol Med on 1 October 2016 by Ah-Koon, L., Lesage, D., et al.
Fig.1.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from J Cell Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 1