Galectin-1 (Gal-1) has been described to promote tumor growth by inducing angiogenesis and to contribute to tumor immune escape by promoting apoptosis of activated T cells. We had previously identified upregulation of Gal-1 in preclinical models of aggressive neuroblastoma (NB), a solid tumor of childhood. However, the clinical and biological relevance of Gal-1 in this tumor entity is unclear. Here, the effect of Gal-1 on the immune system and tumorigenesis was assessed using modulation of Gal-1 expression in immune effector cells and in a transgenic NB model, designated TH-MYCN. The fraction of CD4(+) T cells was decreased in tumor-bearing TH-MYCN mice compared to tumor-free littermates, while both CD4(+) T cells as well as CD8(+) T cells were less activated, compatible with a reduced immune response in tumor-bearing mice. Tumor incidence was not significantly altered by decreasing Gal-1/LGALS1 gene dosage in TH-MYCN mice, but TH-MYCN/Gal-1(-/-) double transgenic mice displayed impaired tumor angiogenesis, splenomegaly, and impaired T cell tumor-infiltration with no differences in T cell activation and apoptosis rate. Additionally, a lower migratory capacity of Gal-1 deficient CD4(+) T cells toward tumor cells was observed in vitro. Transplantation of TH-MYCN-derived tumor cells into syngeneic mice resulted in significantly reduced tumor growth and elevated immune cell infiltration when Gal-1 was downregulated by shRNA. We therefore conclude that T cell-derived Gal-1 mediates T cell tumor-infiltration, whereas NB-derived Gal-1 promotes tumor growth. This opposing effect of Gal-1 in NB should be considered in therapeutic targeting strategies, as currently being developed for other tumor entities.
Product Citations: 4
Immune response modulation by Galectin-1 in a transgenic model of neuroblastoma.
In Oncoimmunology on 1 May 2016 by Büchel, G., Schulte, J. H., et al.
-
FC/FACS
-
Mus musculus (House mouse)
-
Cancer Research
-
Immunology and Microbiology
In Lipids on 1 February 2015 by MacPherson, R. E., Castelli, L. M., et al.
A maternal high fat diet (HFD) can have adverse effects on skeletal muscle development. Skeletal muscle PLIN proteins (PLIN2, 3 and 5) are thought to play critical roles in lipid metabolism, however effects of HFD on PLIN and lipases (HSL, ATGL, CGI-58) in mothers as well as their offspring have yet to be investigated. The primary objective of this study was to determine whether maternal HFD would influence skeletal muscle lipase and PLIN protein content in offspring at weaning (19 d) and young adulthood (3 mo). Female rats (28 d old, n = 9/group) were fed control (CON, AIN93G, 7% soybean oil) or HFD (AIN93G, 20% lard) for 10 weeks prior to mating and throughout pregnancy and lactation. All offspring were weaned to CON [n = 18/group, 1 female and 1 male pup per litter were studied at weaning (19 d) and 3 mo of age]. There was no effect of sex for the main outcomes measured in plantaris, therefore male and female data was combined. Maternal HFD resulted in higher triacylglycerol content in pups at 3 mo (p < 0.05), as well as in the dams (p = 0.015). Maternal HFD resulted in higher PLIN5 content in pups at weaning and 3 mo (p = 0.05). PLIN2 and PLIN5 content decreased at 3 mo versus weaning (p < 0.001). HFD dams had a higher PLIN3 content (p = 0.016). Diet had no effect on ATGL, CGI-58, or HSL content. In conclusion, exposure to a maternal HFD resulted in higher skeletal muscle lipid and PLIN5 content in plantaris of offspring through to young adulthood.
-
Biochemistry and Molecular biology
In Cell Communication and Signaling : CCS on 8 December 2014 by Harder, T., Guttek, K., et al.
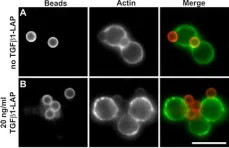
TGFβ1 (Transforming Growth Factor-beta1) is a versatile regulator of T cell immune responses. Depending on its context in the immunological environment, TGFβ1 guides T cells toward specific activation programs including TH17 and regulatory T cell activities. Moreover, TGFβ signals function in immune homeostasis by directly attenuating T cell effector activities. We uncovered a novel context under which TGFβ1 stringently and reversibly silences activation responses of resting human T cells to TCR/CD28 stimulating surfaces:Using ligand-presenting beads, TGFβ1 and TCR/CD28-activating signals were directed into defined plasma membrane domains of T cells. Selective targeting of TGFβ1 cytokine into TCR/CD28 signalling plasma membrane domains held back early response of TCR-proximal tyrosine phosphorylation and bead engulfment at activation sites. Consequently, downstream induction of proliferation and cytokine secretion were stringently attenuated. After extended incubation with TGFβ1-presenting beads, silenced T cells became receptive again to activation by renewed TCR/CD28-stimuli, indicating that the unresponsive state of T cells was reverted and did not reflect long-lasting anergy or decrease in T cell viability. These findings outline a new strategy of physically linking TGFβ1 and TCR-activating functions for the treatment of disease and pathological conditions which are caused by unwanted T cell activity.
-
ICC-IF
-
Homo sapiens (Human)
-
Endocrinology and Physiology
-
Immunology and Microbiology
In Molecular & Cellular Proteomics : MCP on 1 September 2013 by Philipsen, L., Engels, T., et al.
The formation of the immunological synapse between T cells and antigen-presenting cells (APC) begins within minutes of contact and can take hours for full T-cell activation. Although early phases of the synapse have been extensively studied for a select number of proteins, later phases have not yet been examined in detail. We studied the signaling network in stable synapses by measuring the simultaneous localization of 25 signaling and structural molecules over 2 h at the level of individual synapses using multi-epitope ligand cartography (MELC). Signaling proteins including phospho(p)ZAP70, pSLP76, pCD3ζ, and pLAT, along with proteins that influence synapse structure such as F-actin, tubulin, CD45, and ICAM-1, were localized in images of synapses and revealed the multidimensional construction of a mature synapse. The construction of the stable synapse included intense early TCR signaling, a phase of recruitment of structural proteins, and a sustained increase in signaling molecules and colocalization of TCR and pLAT signaling clusters in the center of the synapse. Consolidation of TCR and associated proteins resulted in formation of a small number of discrete synaptic microclusters. Development of synapses and cSMAC composition was greatly affected by the absence of Vav1, with an associated loss in PLCγ1 recruitment, pSLP76, and increased CXCR4. Together, these data demonstrate the use of multi-epitope ligand cartography to quantitatively analyze synapse formation and reveal successive recruitment of structural and signaling proteins and sustained phosphorylation at the mature synapse.
-
WB
-
Mus musculus (House mouse)
-
Biochemistry and Molecular biology
-
Immunology and Microbiology
-
Neuroscience
In Cell Commun Signal on 8 December 2014 by Harder, T., Guttek, K., et al.
Fig.5.A

-
ICC-IF
-
Homo sapiens (Human)
Collected and cropped from Cell Commun Signal by CiteAb, provided under a CC-BY license
Image 1 of 1