Antibody responses, involving B cells, CD4 + T cells, and macrophages, are implicated in autoimmune diseases and organ transplant rejection. We have previously shown that inhibiting FROUNT with disulfiram (DSF) suppresses macrophage activation and migration, effectively treating inflammatory diseases. In this study, we investigated the effectiveness of DSF in antibody-producing reactions. Using a heart transplantation mouse model with antibody-mediated rejection, we administered anti-CD8 antibody to exclude cellular rejection. DSF directly inhibited B cell responses in vitro and significantly reduced plasma donor-specific antibodies and graft antibody deposition in vivo, resulting in prolonged survival of the heart graft. DSF also mediated various effects, including decreased macrophage infiltration and increased Foxp3+ regulatory T-cells in the grafts. Additionally, DSF inhibited pyrimidine metabolism-related gene expression induced by B-cell stimulation. These findings demonstrate that DSF modulates antibody production in the immune response complexity by regulating B-cell and macrophage responses.
© 2024. The Author(s).
Product Citations: 46
In Communications Biology on 22 April 2024 by Chen, W., Toda, E., et al.
-
Mus musculus (House mouse)
-
Biochemistry and Molecular biology
-
Cell Biology
-
Genetics
-
Immunology and Microbiology
Macrophage CD5L is a target for cancer immunotherapy.
In EBioMedicine on 1 May 2023 by Sanchez-Moral, L., Paul, T., et al.
Reprogramming of immunosuppressive tumor-associated macrophages (TAMs) presents an attractive therapeutic strategy in cancer. The aim of this study was to explore the role of macrophage CD5L protein in TAM activity and assess its potential as a therapeutic target.
Monoclonal antibodies (mAbs) against recombinant CD5L were raised by subcutaneous immunization of BALB/c mice. Peripheral blood monocytes were isolated from healthy donors and stimulated with IFN/LPS, IL4, IL10, and conditioned medium (CM) from different cancer cell lines in the presence of anti-CD5L mAb or controls. Subsequently, phenotypic markers, including CD5L, were quantified by flow cytometry, IF and RT-qPCR. Macrophage CD5L protein expression was studied in 55 human papillary lung adenocarcinoma (PAC) samples by IHC and IF. Anti-CD5L mAb and isotype control were administered intraperitoneally into a syngeneic Lewis Lung Carcinoma mouse model and tumor growth was measured. Tumor microenvironment (TME) changes were determined by flow cytometry, IHC, IF, Luminex, RNAseq and RT-qPCR.
Cancer cell lines CM induced an immunosuppressive phenotype (increase in CD163, CD206, MERTK, VEGF and CD5L) in cultured macrophages. Accordingly, high TAM expression of CD5L in PAC was associated with poor patient outcome (Log-rank (Mantel-Cox) test p = 0.02). We raised a new anti-CD5L mAb that blocked the immunosuppressive phenotype of macrophages in vitro. Its administration in vivo inhibited tumor progression of lung cancer by altering the intratumoral myeloid cell population profile and CD4+ T-cell exhaustion phenotype, thereby significantly modifying the TME and increasing the inflammatory milieu.
CD5L protein plays a key function in modulating the activity of macrophages and their interactions within the TME, which supports its role as a therapeutic target in cancer immunotherapy.
For a full list of funding bodies, please see the Acknowledgements.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
-
FC/FACS
-
Cancer Research
-
Immunology and Microbiology
In Frontiers in Immunology on 25 March 2023 by Masle-Farquhar, E., Jeelall, Y., et al.
Germline CARD11 gain-of-function (GOF) mutations cause B cell Expansion with NF-κB and T cell Anergy (BENTA) disease, whilst somatic GOF CARD11 mutations recur in diffuse large B cell lymphoma (DLBCL) and in up to 30% of the peripheral T cell lymphomas (PTCL) adult T cell leukemia/lymphoma (ATL), cutaneous T cell lymphoma (CTCL) and Sezary Syndrome. Despite their frequent acquisition by PTCL, the T cell-intrinsic effects of CARD11 GOF mutations are poorly understood.
Here, we studied B and T lymphocytes in mice with a germline Nethyl-N-nitrosourea (ENU)-induced Card11M365K mutation identical to a mutation identified in DLBCL and modifying a conserved region of the CARD11 coiled-coil domain recurrently mutated in DLBCL and PTCL.
Our results demonstrate that CARD11.M365K is a GOF protein that increases B and T lymphocyte activation and proliferation following antigen receptor stimulation. Germline Card11M365K mutation was insufficient alone to cause B or T-lymphoma, but increased accumulation of germinal center (GC) B cells in unimmunized and immunized mice. Card11M365K mutation caused cell-intrinsic over-accumulation of activated T cells, T regulatory (TREG), T follicular (TFH) and T follicular regulatory (TFR) cells expressing increased levels of ICOS, CTLA-4 and PD-1 checkpoint molecules. Our results reveal CARD11 as an important, cell-autonomous positive regulator of TFH, TREG and TFR cells. They highlight T cell-intrinsic effects of a GOF mutation in the CARD11 gene, which is recurrently mutated in T cell malignancies that are often aggressive and associated with variable clinical outcomes.
Copyright © 2023 Masle-Farquhar, Jeelall, White, Bier, Deenick, Brink, Horikawa and Goodnow.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
Construction of a T cell receptor signaling range for spontaneous development of autoimmune disease.
In The Journal of Experimental Medicine on 6 February 2023 by Tanaka, A., Maeda, S., et al.
Thymic selection and peripheral activation of conventional T (Tconv) and regulatory T (Treg) cells depend on TCR signaling, whose anomalies are causative of autoimmunity. Here, we expressed in normal mice mutated ZAP-70 molecules with different affinities for the CD3 chains, or wild type ZAP-70 at graded expression levels under tetracycline-inducible control. Both manipulations reduced TCR signaling intensity to various extents and thereby rendered those normally deleted self-reactive thymocytes to become positively selected and form a highly autoimmune TCR repertoire. The signal reduction more profoundly affected Treg development and function because their TCR signaling was further attenuated by Foxp3 that physiologically repressed the expression of TCR-proximal signaling molecules, including ZAP-70, upon TCR stimulation. Consequently, the TCR signaling intensity reduced to a critical range generated pathogenic autoimmune Tconv cells and concurrently impaired Treg development/function, leading to spontaneous occurrence of autoimmune/inflammatory diseases, such as autoimmune arthritis and inflammatory bowel disease. These results provide a general model of how altered TCR signaling evokes autoimmune disease.
© 2022 Tanaka et al.
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Mediators of Inflammation on 9 November 2022 by Min, Z., Zhou, J., et al.
The current asthma therapies are inadequate for many patients with severe asthma. Pyrroloquinoline quinone (PQQ) is a naturally-occurring redox cofactor and nutrient that can exert a multitude of physiological effects, including anti-inflammatory and antioxidative effects. We sought to explore the effects of PQQ on allergic airway inflammation and reveal the underlying mechanisms. In vitro, the effects of PQQ on the secretion of epithelial-derived cytokines by house dust mite- (HDM-) incubated 16-HBE cells and on the differentiation potential of CD4+ T cells were investigated. In vivo, PQQ was administered to mice with ovalbumin- (OVA-) induced asthma, and lung pathology and inflammatory cell infiltration were assessed. The changes in T cell subsets and signal transducers and activators of transcription (STATs) were evaluated by flow cytometry. Pretreatment with PQQ significantly decreased HDM-stimulated thymic stromal lymphopoietin (TSLP) production in a dose-dependent manner in 16-HBE cells and inhibited Th2 cell differentiation in vitro. Treatment with PQQ significantly reduced bronchoalveolar lavage fluid (BALF) inflammatory cell counts in the OVA-induced mouse model. PQQ administration also changed the secretion of IFN-γ and IL-4 as well as the percentages of Th1, Th2, Th17, and Treg cells in the peripheral blood and lung tissues, along with inhibition the phosphorylation of STAT1, STAT3, and STAT6 while promoting that of STAT4 in allergic airway inflammation model mice. PQQ can alleviate allergic airway inflammation in mice by improving the immune microenvironment and regulating the JAK-STAT signaling pathway. Our findings suggest that PQQ has great potential as a novel therapeutic agent for inflammatory diseases, including asthma.
Copyright © 2022 Zhihui Min et al.
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
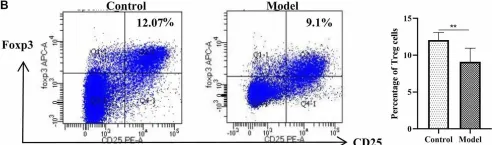
In Front Pharmacol on 7 June 2022 by Zhou, Y., Wang, T., et al.
Fig.3.B

-
FC/FACS
-
Collected and cropped from Front Pharmacol by CiteAb, provided under a CC-BY license
Image 1 of 2
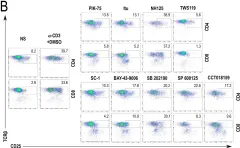
In Sci Rep on 3 July 2018 by Chen, E. W., Brzostek, J., et al.
Fig.4.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 2