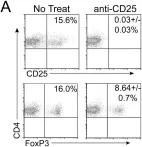
Previously, we found that dysfunctional natural killer (NK) cells with low interferon gamma (IFN-γ) were restored in acute myeloid leukemia (AML) by the FLT4 antagonist MAZ51. Here, we developed 12 peptides targeting FLT4 for clinical application and examined whether they restored the frequency of lymphocytes, especially T cells and NK cells, and high IFN-γ expression, as MAZ51 treatment did in our previous study. Although clinical data from using peptides are currently available, peptides targeting FLT4 to modulate immune cells have not been fully elucidated. In this study, we focus on novel peptide 4 (P4) from the intracellular domain of FLT4 because it had dominant negative activity. Similar to MAZ51, high IFN-γ levels were expressed in AML-mononuclear cells exposed to P4. Additionally, T and NK cell levels were restored, as were high IFN-γ levels, in a leukemic environment when P4 was treated. Interestingly, the regulatory T cells were significantly decreased by P4, implying the role of peptide in tumor niche. Overall, we demonstrated the therapeutic value of functionally modulating lymphocytes using a peptide targeting FLT4 and proposed the development of advanced therapeutic approaches against AML by using immune cells.
© 2023. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Product Citations: 18
Therapeutic potential of FLT4-targeting peptide in acute myeloid leukemia.
In Cancer Immunology, Immunotherapy : CII on 1 September 2023 by Lee, J. Y., Park, S., et al.
-
Mus musculus (House mouse)
-
Cancer Research
Therapeutic potential of FLT4-targeting peptide in acute myeloid leukemia
Preprint on Research Square on 4 October 2022 by Lee, J. Y., Park, S., et al.
Fms-related tyrosine kinase-4 (FLT4) is involved in tumor progression. Previously, we found that dysfunctional natural killer (NK) cells with low interferon-gamma (IFN-γ) were restored in acute myeloid leukemia (AML) by the FLT4 antagonist MAZ51. In this work, we developed 12 peptides targeting FLT4 for clinical application and ultimately selected 4 of them to examine whether they restored the frequency of lymphocytes, especially T cells and NK cells, and high IFN-γ expression, as MAZ51 treatment did in our previous study. Although clinical data from using intracellular kinase domain–targeting peptides are currently available, peptides targeting FLT4 to modulate immune cells have not been fully elucidated. In this study, we focus on novel peptide 4 (P4) from the intracellular domain of FLT4 because it had dominant negative activity. Similar to MAZ51, high IFN-γ levels were expressed in AML-mononuclear cells (MNCs) exposed to P4. In addition, T and NK cell levels were restored, as were high IFN-γ levels, in a leukemic environment when P4 was co-cultured with cytosine β-D-arabinofuranoside. Interestingly, the frequency of regulatory T cells was significantly decreased by P4, implying that the peptide plays a role in modulating the tumor niche. Overall, we demonstrated the therapeutic value of functionally modulating lymphocytes using a peptide targeting FLT4 and propose the development of advanced therapeutic approaches against AML by using immune cells.
-
Mus musculus (House mouse)
-
Cancer Research
In Cell Reports on 15 March 2022 by Fernández-Pisonero, I., Clavain, L., et al.
A missense change in RRAS2 (Gln72 to Leu), analogous to the Gln61-to-Leu mutation of RAS oncoproteins, has been identified as a long-tail hotspot mutation in cancer and Noonan syndrome. However, the relevance of this mutation for in vivo tumorigenesis remains understudied. Here we show, using an inducible knockin mouse model, that R-Ras2Q72L triggers rapid development of a wide spectrum of tumors when somatically expressed in adult tissues. These tumors show limited overlap with those originated by classical Ras oncogenes. R-Ras2Q72L-driven tumors can be classified into different subtypes according to therapeutic susceptibility. Importantly, the most relevant R-Ras2Q72L-driven tumors are dependent on mTORC1 but independent of phosphatidylinositol 3-kinase-, MEK-, and Ral guanosine diphosphate (GDP) dissociation stimulator. This pharmacological vulnerability is due to the extensive rewiring by R-Ras2Q72L of pathways that orthogonally stimulate mTORC1 signaling. These findings demonstrate that RRAS2Q72L is a bona fide oncogenic driver and unveil therapeutic strategies for patients with cancer and Noonan syndrome bearing RRAS2 mutations.Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
-
Mus musculus (House mouse)
-
Cancer Research
Cancer-associated mutations in VAV1 trigger variegated signaling outputs and T-cell lymphomagenesis.
In The EMBO Journal on 15 November 2021 by Robles-Valero, J., Fernández-Nevado, L., et al.
Mutations in VAV1, a gene that encodes a multifunctional protein important for lymphocytes, are found at different frequencies in peripheral T-cell lymphoma (PTCL), non-small cell lung cancer, and other tumors. However, their pathobiological significance remains unsettled. After cataloguing 51 cancer-associated VAV1 mutations, we show here that they can be classified in five subtypes according to functional impact on the three main VAV1 signaling branches, GEF-dependent activation of RAC1, GEF-independent adaptor-like, and tumor suppressor functions. These mutations target new and previously established regulatory layers of the protein, leading to quantitative and qualitative changes in VAV1 signaling output. We also demonstrate that the most frequent VAV1 mutant subtype drives PTCL formation in mice. This process requires the concurrent engagement of two downstream signaling branches that promote the chronic activation and transformation of follicular helper T cells. Collectively, these data reveal the genetic constraints associated with the lymphomagenic potential of VAV1 mutant subsets, similarities with other PTCL driver genes, and potential therapeutic vulnerabilities.
© 2021 The Authors. Published under the terms of the CC BY NC ND 4.0 license.
-
FC/FACS
-
Mus musculus (House mouse)
-
Cancer Research
-
Immunology and Microbiology
In Frontiers in Cell and Developmental Biology on 18 December 2020 by Li, Y., Ma, K., et al.
Inflammatory bowel disease (IBD), which main clinical manifestations include abdominal pain and diarrhea occurring repeatedly, is a kind of autoimmune disease. It has been reported in preceding studies that mesenchymal stem cells (MSCs) can reduce inflammation by regulating the function of immune cells. But studies about the interaction between MSCs and adaptive immune cells, especially in IBD models, are insufficient. Therefore, the objective of this research was to estimate the therapeutic effects of MSCs from human umbilical cord blood (hUCB-MSCs) in an IBD model of rodent and to clarify the therapeutic mechanisms of hUCB-MSCs. Dextran sulfate sodium (DSS) was used to induce colitis in rodent. Mice with colitis were treated with intraperitoneal infusions of hUCB-MSCs and evaluated for mortality and diverse disease symptoms containing weight reduction, diarrhea, and bloody stools. The levels of histopathologic severity and generation of regulatory T cells (Treg) were also determined. Treatment with hUCB-MSCs ameliorated the clinical and histopathologic severity of acute and chronic colitis in mice. Furthermore, T cell infiltration into the inflamed colon was significantly decreased (p = 0.0175), and Foxp3+ cells were substantially higher in the hUCB-MSC group than that of the DSS group. Our results suggest that hUCB-MSCs are able to alleviate inflammation via adding Foxp3+ Tregs in an IBD model of mouse. As a result, these findings suggest the opportunity of hUCB-MSC being applied to patients with IBD.
Copyright © 2020 Li, Ma, Zhang, Xu and Zhang.
-
FC/FACS
-
Mus musculus (House mouse)
-
Cardiovascular biology
-
Immunology and Microbiology
-
Stem Cells and Developmental Biology
In PLoS One on 3 November 2011 by Côté, A. L., Byrne, K. T., et al.
Fig.2.A

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 1