Antigen encounter directs CD4+ T cells to differentiate into T helper or regulatory cells. This process focuses the immune response on the invading pathogen and limits tissue damage. Mechanisms that govern T helper cell versus T regulatory cell fate remain poorly understood. Here, we show that the E3 ubiquitin ligase Cul5 determines fate selection in CD4+ T cells by regulating IL-4 receptor signaling. Mice lacking Cul5 in T cells develop Th2 and Th9 inflammation and show pathophysiological features of atopic asthma. Following T cell activation, Cul5 forms a complex with CIS and pJak1. Cul5 deletion reduces ubiquitination and subsequent degradation of pJak1, leading to an increase in pJak1 and pSTAT6 levels and reducing the threshold of IL-4 receptor signaling. As a consequence, Cul5 deficient CD4+ T cells deviate from Treg to Th9 differentiation in low IL-4 conditions. These data support the notion that Cul5 promotes a tolerogenic T cell fate choice and reduces susceptibility to allergic asthma.
© 2022. The Author(s).
Product Citations: 367
The ubiquitin ligase Cul5 regulates CD4+ T cell fate choice and allergic inflammation.
In Nature Communications on 19 May 2022 by Kumar, B., Field, N. S., et al.
-
Immunology and Microbiology
-
Stem Cells and Developmental Biology
Preprint on BioRxiv : the Preprint Server for Biology on 31 December 2021 by Kane, H., LaMarche, N. M., et al.
Innate T cells, including CD1d-restricted invariant natural killer T (iNKT) cells, are characterized by their rapid activation in response to non-peptide antigens, such as lipids. While the transcriptional profiles of naive, effector and memory adaptive T cells have been well studied, less is known about transcriptional regulation of different iNKT cell activation states. Here, using single cell RNA-sequencing, we performed longitudinal profiling of activated iNKT cells, generating a transcriptomic atlas of iNKT cell activation states. We found that transcriptional signatures of activation are highly conserved among heterogeneous iNKT cell populations, including NKT1, NKT2 and NKT17 subsets, and human iNKT cells. Strikingly, we found that regulatory iNKT cells, such as adipose iNKT cells, undergo blunted activation, and display constitutive enrichment of memory-like cMAF + and KLRG1 + populations. Moreover, we identify a conserved cMAF-associated transcriptional network among NKT10 cells, providing novel insights into the biology of regulatory and antigen experienced iNKT cells.
-
Biochemistry and Molecular biology
-
Immunology and Microbiology
In Autophagy on 1 September 2021 by Oh, D. S., Park, J. H., et al.
Respiratory syncytial virus (RSV) is a leading cause of respiratory tract infections in infants. Macroautophagy/autophagy is a catalytic metabolic process required for cellular homeostasis. Although intracellular metabolism is important for immune responses in dendritic cells, the link between autophagy and immunometabolism remains unknown. Here, we show that the autophagy-related protein ATG5 regulates immunometabolism. Atg5-deficient mouse dendritic cells showed increased CD8A+ T-cell response and increased secretion of proinflammatory cytokines upon RSV infection. Transcriptome analysis showed that Atg5 deficiency alters the expression of metabolism-related genes. Atg5-deficient dendritic cells also showed increased activation of glycolysis and the AKT-MTOR-RPS6KB1 pathway and decreased mitochondrial activity, all of which are cellular signatures for metabolic activation. These cells also showed elevated CD8A+ T-cell priming and surface major histocompatibility complex (MHC) class I expression. Our results suggested that ATG5 regulated host immune responses by modulating dendritic cell metabolism. These findings may help develop potential antiviral therapies that alter host immunity by regulating autophagy and immunometabolism.Abbreviations : 2-DG: 2-deoxyglucose; AAK1: AP2 associated kinase 1; AKT: AKT serine/threonine kinase; AM: alveolar macrophage; ATG: autophagy; ATP: adenosine triphosphate; BAL: bronchoalveolar lavage; BMDC: bone marrow dendritic cell; CSF2/GM-CSF: colony-stimulating factor 2 (granulocyte-macrophage); CTL: cytotoxic T lymphocyte; ELISA: enzyme-linked immunosorbent assay; GFP: green fluorescent protein; GSEA: gene-set enrichment analysis; H-2Db: H-2 class I histocompatibility antigen, D-B alpha chain; H-2Kb: MHC class I H2-K-b; HIF1A: hypoxia-inducible factor 1 alpha; IFNG: interferon-gamma; IL: interleukin; ITGAX: integrin alpha X; MAP1LC3/LC3: microtubule-associated protein 1 light chain 3; MAP1LC3B/LC3B: microtubule-associated protein 1 light chain 3 beta; MHC: major histocompatibility complex; MTORC1: mammalian target of rapamycin kinase complex 1; PBS: phosphate-buffered saline; PFU: plaque-forming unit; RLR: retinoic acid-inducible-I-like receptor; ROS: reactive oxygen species; RPMI: Roswell Park Memorial Institute; RPS6KB1/S6K: ribosomal protein S6 kinase, polypeptide 1; RSV: respiratory syncytial virus; Th: T helper; TLR: toll-like receptor; Treg: regulatory T cells; UMAP: uniform manifold approximation and projection.
-
Cell Biology
-
Immunology and Microbiology
In Protein Cell on 1 August 2021 by Sun, Y., Wang, Q., et al.
Axonal degeneration is one of the key features of neurodegenerative disorders. In the canonical view, axonal degeneration destructs neural connections and promotes detrimental disease defects. Here, we assessed the enteric nervous system (ENS) of the mouse, non-human primate, and human by advanced 3D imaging. We observed the profound neurodegeneration of catecholaminergic axons in human colons with ulcerative colitis, and similarly, in mouse colons during acute dextran sulfate sodium-induced colitis. However, we unexpectedly revealed that blockage of such axonal degeneration by the Sarm1 deletion in mice exacerbated the colitis condition. In contrast, pharmacologic ablation or chemogenetic inhibition of catecholaminergic axons suppressed the colon inflammation. We further showed that the catecholaminergic neurotransmitter norepinephrine exerted a pro-inflammatory function by enhancing the expression of IL-17 cytokines. Together, this study demonstrated that Sarm1-mediated neurodegeneration within the ENS mitigated local inflammation of the colon, uncovering a previously-unrecognized beneficial role of axonal degeneration in this disease context.
© 2021. The Author(s).
-
Immunology and Microbiology
-
Neuroscience
In Nature Communications on 22 July 2021 by Koda, Y., Teratani, T., et al.
Non-alcoholic steatohepatitis (NASH) is a leading cause of chronic liver disease that can progress to liver fibrosis. Recent clinical advance suggests a reversibility of liver fibrosis, but the cellular and molecular mechanisms underlying NASH resolution remain unclarified. Here, using a murine diet-induced NASH and the subsequent resolution model, we demonstrate direct roles of CD8+ tissue-resident memory CD8+ T (CD8+ Trm) cells in resolving liver fibrosis. Single-cell transcriptome analysis and FACS analysis revealed CD69+CD103-CD8+ Trm cell enrichment in NASH resolution livers. The reduction of liver CD8+ Trm cells, maintained by tissue IL-15, significantly delayed fibrosis resolution, while adoptive transfer of these cells protected mice from fibrosis progression. During resolution, CD8+ Trm cells attracted hepatic stellate cells (HSCs) in a CCR5-dependent manner, and predisposed activated HSCs to FasL-Fas-mediated apoptosis. Histological assessment of patients with NASH revealed CD69+CD8+ Trm abundance in fibrotic areas, further supporting their roles in humans. These results highlight the undefined role of liver CD8+ Trm in fibrosis resolution.
© 2021. The Author(s).
-
Immunology and Microbiology
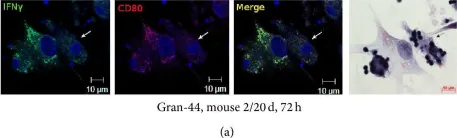
In J Immunol Res on 12 April 2016 by Ufimtseva, E.
Fig.6.A

-
ICC-IF
-
Mus musculus (House mouse)
Collected and cropped from J Immunol Res by CiteAb, provided under a CC-BY license
Image 1 of 4
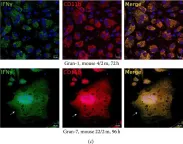
In J Immunol Res on 12 April 2016 by Ufimtseva, E.
Fig.6.C

-
ICC-IF
-
Mus musculus (House mouse)
Collected and cropped from J Immunol Res by CiteAb, provided under a CC-BY license
Image 1 of 4
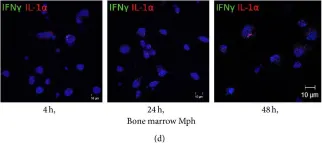
In J Immunol Res on 12 April 2016 by Ufimtseva, E.
Fig.6.D

-
ICC-IF
-
Mus musculus (House mouse)
Collected and cropped from J Immunol Res by CiteAb, provided under a CC-BY license
Image 1 of 4
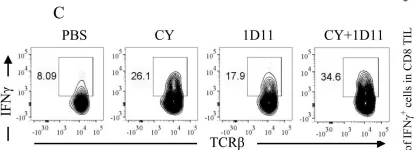
In PLoS One on 15 January 2014 by Chen, X., Yang, Y., et al.
Fig.4.C

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 4