Mesenchymal stem cells (MSCs) are frequently used for therapeutic applications in both pre-clinical and clinical settings owing to their capacity for immune modulation and neuroprotective effects. However, transient fever is commonly observed as an adverse event following MSC injection in patients with Alzheimer's disease (AD). In this study, we investigated the potential impact of immunosuppressants such as dexamethasone and tacrolimus on altering the characteristics of human mesenchymal stem cells (hMSCs). Additionally, we examined whether these immunosuppressants affect the persistence of hMSCs or the immune response upon their administration into the brain parenchyma of AD mice. The exposure of hMSCs to high concentrations of dexamethasone and tacrolimus in vitro did not significantly alter the characteristics of hMSCs. The expression of genes related to innate immune responses, such as Irak1, Irf3, Nod1, and Ifnar1, was significantly downregulated by the additional administration of dexamethasone and tacrolimus to the brain parenchyma of AD mice. However, hMSC persistence in the AD mouse brain was not affected. The results of this study support the use of immunosuppressants to mitigate fever during stem cell therapy in patients with AD.
Product Citations: 87
In International Journal of Stem Cells on 30 May 2025 by Lee, N. K., Na, D. L., et al.
-
Stem Cells and Developmental Biology
In Stem Cells Translational Medicine on 15 July 2024 by Tesch, R. S., Takamori, E. R., et al.
Condylar resorption is an aggressive and disability form of temporomandibular joint (TMJ) degenerative disease, usually non-respondent to conservative or minimally invasive therapies and often leading to surgical intervention and prostheses implantation. This condition is also one of the most dreaded postoperative complications of orthognathic surgery, with severe cartilage erosion and loss of subchondral bone volume and mineral density, associated with a painful or not inflammatory processes. Because regenerative medicine has emerged as an alternative for orthopedic cases with advanced degenerative joint disease, we conducted a phase I/IIa clinical trial (U1111-1194-6997) to evaluate the safety and efficacy of autologous nasal septal chondroprogenitor cells. Ten participants underwent biopsy of the nasal septum cartilage during their orthognathic surgery. The harvested cells were cultured in vitro and analyzed for viability, presence of phenotype markers for mesenchymal stem and/or chondroprogenitor cells, and the potential to differentiate into chondrocytes, adipocytes, and osteoblasts. After the intra-articular injection of the cell therapy, clinical follow-up was performed using the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) and computed tomography (CT) images. No serious adverse events related to the cell therapy injection were observed during the 12-month follow-up period. It was found that autologous chondroprogenitors reduced arthralgia, promoted stabilization of mandibular function and condylar volume, and regeneration of condylar tissues. This study demonstrates that chondroprogenitor cells from the nasal septum may be a promise strategy for the treatment of temporomandibular degenerative joint disease that do not respond to other conservative therapies.
© The Author(s) 2024. Published by Oxford University Press.
-
Stem Cells and Developmental Biology
In Cellular and Molecular Life Sciences : CMLS on 11 July 2024 by Kushida, Y., Oguma, Y., et al.
Muse cells, identified as cells positive for the pluripotent surface marker SSEA-3, are pluripotent-like endogenous stem cells located in the bone marrow (BM), peripheral blood, and organ connective tissues. The detailed characteristics of SSEA-3(+) cells in extraembryonic tissue, however, are unknown. Here, we demonstrated that similar to human-adult tissue-Muse cells collected from the BM, adipose tissue, and dermis as SSEA-3(+), human-umbilical cord (UC)-SSEA-3(+) cells express pluripotency markers, differentiate into triploblastic-lineage cells at a single cell level, migrate to damaged tissue, and exhibit low telomerase activity and non-tumorigenicity. Notably, ~ 20% of human-UC-SSEA-3(+) cells were negative for X-inactive specific transcript (XIST), a naïve pluripotent stem cell characteristic, whereas all human adult tissue-Muse cells are XIST-positive. Single-cell RNA sequencing revealed that the gene expression profile of human-UC-SSEA-3(+) cells was more similar to that of human post-implantation blastocysts than human-adult tissue-Muse cells. The DNA methylation level showed the same trend, and notably, the methylation levels in genes particularly related to differentiation were lower in human-UC-SSEA-3(+) cells than in human-adult tissue-Muse cells. Furthermore, human-UC-SSEA-3(+) cells newly express markers specific to extraembryonic-, germline-, and hematopoietic-lineages after differentiation induction in vitro whereas human-adult tissue-Muse cells respond only partially to the induction. Among various stem/progenitor cells in living bodies, those that exhibit properties similar to post-implantation blastocysts in a naïve state have not yet been found in humans. Easily accessible human-UC-SSEA-3(+) cells may be a valuable tool for studying early-stage human development and human reproductive medicine.
© 2024. The Author(s).
-
Biochemistry and Molecular biology
Preprint on Research Square on 20 June 2024 by Kushida, Y., Oguma, Y., et al.
Abstract Muse cells, identified as cells positive for the pluripotent surface marker SSEA-3, are pluripotent-like endogenous stem cells located in the bone marrow (BM), peripheral blood, and organ connective tissues. The detailed characteristics of SSEA-3(+) cells in extraembryonic tissue, however, are unknown. Here, we demonstrated that similar to human-adult tissue-Muse cells collected from the BM, adipose tissue, and dermis as SSEA-3(+), human-umbilical cord (UC)-SSEA-3(+) cells express pluripotency markers, differentiate into triploblastic-lineage cells at a single cell level, migrate to damaged tissue, and exhibit low telomerase activity and non-tumorigenicity. Notably, ~ 20% of human-UC-SSEA-3(+) cells were negative for X-inactive specific transcript (XIST), a naïve pluripotent stem cell characteristic, whereas all human adult tissue-Muse cells are XIST-positive. Single-cell RNA sequencing revealed that the gene expression profile of human-UC-SSEA-3(+) cells was more similar to that of human post-implantation blastocysts than human-adult tissue-Muse cells. The DNA methylation level showed the same trend, and notably, the methylation levels in genes particularly related to differentiation were lower in human-UC-SSEA-3(+) cells than in human-adult tissue-Muse cells. Furthermore, human-UC-SSEA-3(+) cells newly express markers specific to extraembryonic-, germline-, and hematopoietic-lineages after differentiation induction in vitro whereas human-adult tissue-Muse cells respond only partially to the induction. Among various stem/progenitor cells in living bodies, those that exhibit properties similar to post-implantation blastocysts in a naïve state have not yet been found in humans. Easily accessible human-UC-SSEA-3(+) cells may be a valuable tool for studying early-stage human development and human reproductive medicine.
In Polymers on 29 September 2023 by Pérez-Díaz, M. A., Martínez-Colin, E. J., et al.
Cross-linked polymer blends from natural compounds, namely gelatin (Gel), chitosan (CS), and synthetic poly (vinyl alcohol) (PVA), have received increasing scrutiny because of their versatility, biocompatibility, and ease of use for tissue engineering. Previously, Gel/CS/PVA [1:1:1] hydrogel produced via the freeze-drying process presented enhanced mechanical properties. This study aimed to investigate the biocompatibility and chondrogenic potential of a steam-sterilized Gel/CS/PVA hydrogel using differentiation of human adipose-derived mesenchymal stromal cells (AD-hMSC) and cartilage marker expression. AD-hMSC displayed fibroblast-like morphology, 90% viability, and 69% proliferative potential. Mesenchymal profiles CD73 (98.3%), CD90 (98.6%), CD105 (97.0%), CD34 (1.11%), CD45 (0.27%), HLA-DR (0.24%); as well as multilineage potential, were confirmed. Chondrogenic differentiation of AD-hMSC in monolayer revealed the formation of cartilaginous nodules composed of glycosaminoglycans after 21 days. Compared to nonstimulated cells, hMSC-derived chondrocytes shifted the expression of CD49a from 2.82% to 40.6%, CD49e from 51.4% to 92.2%, CD54 from 9.66 to 37.2%, and CD151 from 45.1% to 75.8%. When cultured onto Gel/CS/PVA hydrogel during chondrogenic stimulation, AD-hMSC changed to polygonal morphology, and chondrogenic nodules increased by day 15, six days earlier than monolayer-differentiated cells. SEM analysis showed that hMSC-derived chondrocytes adhered to the surface with extended filopodia and abundant ECM formation. Chondrogenic nodules were positive for aggrecan and type II collagen, two of the most abundant components in cartilage. This study supports the biocompatibility of AD-hMSC onto steam-sterilized GE/CS/PVA hydrogels and its improved potential for chondrocyte differentiation. Hydrogel properties were not altered after steam sterilization, which is relevant for biosafety and biomedical purposes.
-
FC/FACS
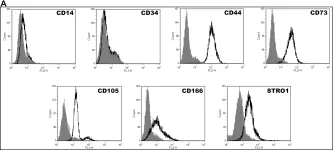
In PLoS One on 30 August 2014 by Tsai, H. L., Deng, W. P., et al.
Fig.1.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 1