m6A RNA methylation is essential for many aspects of mammalian development but its roles in chondrogenesis remain largely unknown. Here, we show that m6A is necessary for chondrogenesis and limb morphogenesis using limb progenitor-specific knockout mice of Mettl14, an essential subunit in the m6A methyltransferase complex. The knockout disrupts cartilage anlagen formation in limb buds with 11 downregulated proteins known to dysregulate chondrogenesis and shorten limb skeletons upon mutation in mice and humans. Further studies show a gene regulatory hierarchy among the 11 proteins. m6A stabilizes the transcript and increases the protein level of GDF5, a BMP family member. This activates the chondrogenic transcription factor genes Runx2 and Runx3, whose mRNAs are also stabilized by m6A. They promote the transcription of six collagen genes and two other chondrogenic genes, Ddrgk1 and Pbxip1. Thus, this study uncovers an m6A-based cascade essential for chondrogenesis during limb skeletal development.
© 2025. The Author(s).
Product Citations: 479
In Nature Communications on 30 April 2025 by Katoku-Kikyo, N., Kawakami, H., et al.
In Biology of the Cell / Under the Auspices of the European Cell Biology Organization on 1 April 2025 by Thetiot, M., Sart, S., et al.
Neural stem cells (NSCs) generate neurons and glia in the adult vertebrate brain, crucial for tissue maintenance and plasticity. They balance neurogenesis with self-renewal, regulated through transitions between quiescence, activation, and lineage progression. The molecular and cellular mechanisms behind these processes remain incompletely understood.
Here, we describe a protocol to isolate and expand NSCs from the adult zebrafish pallium, a major NSC niche. We present the procedures to propagate primary cultures of NSCs, followed by the generation of 3D spheres and their regulation in a droplet microfluidic platform. We then detail the procedure to analyze adult NSC fate within the 3D spheroids following drug treatment.
We show that 7 µL droplets are sufficient to allow the formation of size-controlled 3D spheroids, in which NSCs sustain self-renewal and are able to balance quiescence and activation. We outline potential applications, including investigation of factors involved in adult NSC activation and monitoring of their soluble environment, for which a confined culture system is advantageous.
© 2025 Société Française des Microscopies and Société de Biologie Cellulaire de France. Published by John Wiley & Sons Ltd.
-
Cell Biology
-
Stem Cells and Developmental Biology
Pulmonary lymphoid tissue induced after SARS-CoV-2 infection in rhesus macaques.
In Frontiers in Immunology on 27 March 2025 by Ma, Z. M., Olstad, K. J., et al.
Lung diseases are widespread worldwide. Pulmonary immunity plays a vital role against lung pathogens, including SARS-CoV-2 infection. Understanding the pathogenesis, including the development of local immune responses to infection, is fundamental for developing interventions to control the viral infection.
Using immunohistochemistry, we investigated the distribution of immune cells in the lungs of rhesus macaques experimentally infected with SARS-CoV-2 and euthanized 11-14 days later.
Tertiary lymphoid tissue was found in all SARS-CoV-2 infected animals. The number (13.9 vs 1.5 iPLT number/ lung cm2), size (25992 vs 13946 µm2) and total area (0.46 vs 0.02 mm2 iPLT/ lung cm2) of the lymphoid tissue aggregations were significantly higher in SARS-CoV-2 infected animals than that of normal controls. This induced pulmonary lymphoid tissues comprised B cells, T cells, CD169 macrophages, and follicular dendritic cells with evidence of lymphocyte priming and differentiation.
The results suggest local immunity plays an important role in the SARS-CoV-2 infection. Further study of pulmonary immunity could lead to new interventions to develop vaccine strategies and discover new immune-regulatory biomarkers in monitoring and controlling SARS-CoV-2 infection and other lung diseases.
Copyright © 2025 Ma, Olstad, Van Rompay, Iyer, Miller and Reader.
-
Cardiovascular biology
-
COVID-19
-
Immunology and Microbiology
In Theranostics on 14 March 2025 by Lee, J. Y., Na, Y. R., et al.
Superparamagnetic iron oxide nanoparticles (SPIONs) are promising contrast agents for imaging-guided cancer therapies. However, challenges such as the requirement for a high alternating magnetic field (AMF), dosage limitations, and suboptimal imaging contrast have hindered their practical applications. Methods: First, the optimal doping ratio of Mn and Zn in MnxZn1-xFe2O4 nanoparticles synthesized using a modified high-temperature thermal decomposition method (mHTTD) was determined. Then, the magnetic and physical properties of the optimal 7-nm Mn0.5Zn0.5Fe2O4 SPIONs were systematically and comprehensively characterized via hysteresis measurements, dynamic light scattering (DLS), transmission electron microscopy (TEM), X-ray diffraction (XRD), X-ray absorption fine structure (XAFS) spectroscopy, and X-ray absorption near edge structure (XANES) spectroscopy. Next, the stability, biosafety, biocompatibility, and theranostic performance of 7-nm Mn0.5Zn0.5Fe2O4 SPIONs in magnetic hyperthermia therapy (MHT) were evaluated by in vivo and in vitro studies involving mouse models, magnetic resonance imaging (MRI), and bioassays. The results were then compared with those for conventional SPIONs. Results: Under an AMF of 140 Oe at 100 kHz, 7-nm Mn0.5Zn0.5Fe2O4 SPIONs demonstrated significantly higher heat production than conventional SPIONs. Following surface modification with methoxy-PEG-silane, PEGylated 7-nm Mn0.5Zn0.5Fe2O4 SPIONs showed excellent monodispersity and magnetic properties, with an exceptionally high T2 relaxivity (r2). Conclusions: The high in vitro and in vivo theranostic performance of PEGylated 7-nm Mn0.5Zn0.5Fe2O4 SPIONs as efficient and stable contrast agents for treating glioblastoma, encompassing strengthened magnetic hyperthermia, activated anti-tumor immunity, and remarkable T2 contrast enhancement, underscores the potential of precisely designed ferrites to concurrently enhance the T2 contrast and magnetocaloric properties for optimal theranostic outcomes. Our study provides a compelling rationale for the development of tailored magnetic nanoprobes for improved glioblastoma theranostics.
© The author(s).
In International Journal of Nanomedicine on 4 March 2025 by Carrasco-Díaz, L. M., Gallardo, A., et al.
Colorectal cancer (CRC) has traditionally been treated with genotoxic chemotherapy to activate pro-apoptotic proteins to induce anticancer effects. However, cancer cells develop resistance to apoptosis, which leads to recurrence and poor prognosis. Moreover, this kind of therapy has been shown to be highly toxic to healthy tissues and, therefore, to patients. To overcome this issue, we developed a self-assembly tumor-targeted nanoparticle, T22-DITOX-H6, that incorporates the T22 peptide (a CXCR4 ligand) to selectively target cells overexpressing CXCR4, fused to the catalytic domain of diphtheria toxin, that exhibits a potent cytotoxic effect on these CXCR4+ cancer cells that exhibits potent cytotoxic effects on CXCR4-overexpressing cancer cells through the activation of pyroptosis, an immunogenic type of cell death.
Colorectal CXCR4-expressing tumor cells (CT26-CXCR4+) were implanted subcutaneously into immunocompetent mice to study the effects of T22-DITOX-H6 treatment on tumor growth, cell death and innate immune cell recruitment to the tumor.
Here, we demonstrated that the T22-DITOX-H6 nanoparticle selectively activated pyroptosis, an immunogenic cell death that differs from apoptosis, leading to cell death in CXCR4-expressing cells, without affecting the viability of CXCR4-lacking cells. In addition, the nanoparticle administered to tumor-bearing mice induced a local antitumor effect due to the selective activation of pyroptosis in CXCR4+ targeted cancer cells. Biochemical analysis of plasma and histological analysis of non-tumor tissues revealed no differences between the groups. Remarkably, pyroptosis activation stimulates eosinophil infiltration into the tumor microenvironment, an effect recently reported to have an anti-tumorigenic function.
These results highlight the dual role of CXCR4-targeted cytotoxic nanoparticle in eliminating cancer cells and boosting the self-immune response without compromising healthy organs.
© 2025 Carrasco-Díaz et al.
-
Cancer Research
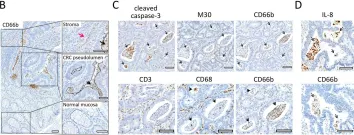
In Cell Death Dis on 4 February 2022 by Schimek, V., Strasser, K., et al.
Fig.4.C

-
IHC
-
Homo sapiens (Human)
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 2
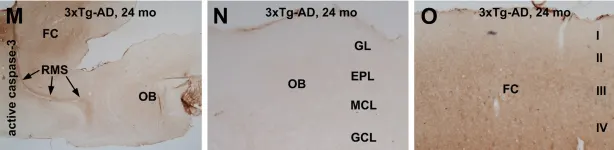
In PLoS One on 23 June 2012 by Cai, Y., Zhu, H. X., et al.
Fig.6.M,N,O

-
IHC
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 2