Tertiary lymphoid structures (TLSs) are de novo ectopic lymphoid aggregates that regulate immunity in chronically inflamed tissues, including tumours. Although TLSs form due to inflammation-triggered activation of the lymphotoxin (LT)-LTβ receptor (LTβR) pathway1, the inflammatory signals and cells that induce TLSs remain incompletely identified. Here we show that interleukin-33 (IL-33), the alarmin released by inflamed tissues2, induces TLSs. In mice, Il33 deficiency severely attenuates inflammation- and LTβR-activation-induced TLSs in models of colitis and pancreatic ductal adenocarcinoma (PDAC). In PDAC, the alarmin domain of IL-33 activates group 2 innate lymphoid cells (ILC2s) expressing LT that engage putative LTβR+ myeloid organizer cells to initiate tertiary lymphoneogenesis. Notably, lymphoneogenic ILC2s migrate to PDACs from the gut, can be mobilized to PDACs in different tissues and are modulated by gut microbiota. Furthermore, we detect putative lymphoneogenic ILC2s and IL-33-expressing cells within TLSs in human PDAC that correlate with improved prognosis. To harness this lymphoneogenic pathway for immunotherapy, we engineer a recombinant human IL-33 protein that expands intratumoural lymphoneogenic ILC2s and TLSs and demonstrates enhanced anti-tumour activity in PDAC mice. In summary, we identify the molecules and cells of a druggable pathway that induces inflammation-triggered TLSs. More broadly, we reveal a lymphoneogenic function for alarmins and ILC2s.
© 2025. The Author(s).
Product Citations: 23
IL-33-activated ILC2s induce tertiary lymphoid structures in pancreatic cancer.
In Nature on 1 February 2025 by Amisaki, M., Zebboudj, A., et al.
-
Cancer Research
Concomitant loss of TET2 and TET3 results in T cell expansion and genomic instability in mice.
In Communications Biology on 3 December 2024 by Gioulbasani, M., Äijö, T., et al.
Ten eleven translocation (TET) proteins are tumor suppressors that through their catalytic activity oxidize 5-methylcytosine to 5-hydroxymethylcytosine, to promote DNA demethylation and to regulate gene expression. Notably, TET2 is one of the most frequently mutated genes in hematological malignancies, including T cell lymphomas. However, murine models with deletion of TET2 do not exhibit T cell expansion, presumably due to redundancy with other members of the TET family of proteins. In order to gain insight on the TET mediated molecular events that safeguard T cells from aberrant proliferation we performed serial adoptive transfers of murine CD4 T cells that lack concomitantly TET2 and TET3 to fully immunocompetent congenic mice. Here we show a progressive acquisition of malignant traits upon loss of TET2 and TET3 that is characterized by loss of genomic integrity, acquisition of aneuploidy and upregulation of the protooncogene Myc.
© 2024. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
RepeatAscarischallenge reduces worm intensity through gastric cellular reprograming
Preprint on BioRxiv : the Preprint Server for Biology on 29 August 2024 by Wu, Y., Suarez-Reyes, C., et al.
Ascariasis (roundworm) is the most prevalent parasitic nematode infection worldwide, impacting approximately 500 million people predominantly in low- and middle-income countries (LMICs). While people of all ages are infected with Ascaris , infection intensity (defined by worm burden) paradoxically peaks in pre-school and school aged children but then declines with age. The cause of age-dependent Ascaris worm intensity is not well understood but may be dependent on cellular changes in mucosal barrier sites. We have previously found that the gastric mucosa is a critical barrier site for Ascaris infection. Following oral ingestion of Ascaris eggs, larvae use AMCase secreted by gastric chief cells and acid secreted by gastric parietal cells to hatch. Once hatched, larvae translocate across the gastric mucosa to initiate the larval migratory cycle. However, inducing mucosal injury with administration of Tamoxifen induces mucosa cellular changes that impairs Ascaris hatching and reduces larval translocation across the gastric mucosa. In this study we established a repeated Ascaris suum challenge mouse model and evaluated if repeated Ascaris challenge also lead to cellular changes in the gastric mucosal barrier. We found that repeated Ascaris challenge caused cellular changes in the gastric mucosa which reduced worm intensity in the liver independent of the adaptive immune response. Thus, in endemic regions, where individuals experience recurrent infection throughout their lives, gastric cellular changes may be a key mechanism leading to the observed age-dependent Ascaris worm intensity changes from childhood to adulthood.
In Poultry Science on 1 June 2024 by Hou, Q., Li, G., et al.
Aging is associated with alterations in gut function, including intestinal inflammation, leaky gut, and impaired epithelial regeneration. Rejuvenating the aged gut is imperative to extend the laying cycle of aged laying hens. Genistein is known to have beneficial effects on age-related diseases, but its precise role in homeostasis of the aged gut of laying hens remains to be elucidated. In this study, 160 45-wk-old Hyline Brown laying hens were continuously fed a basal diet or a diet supplemented with 40 mg/kg genistein until they reached 100 wk of age. The results revealed that long-term genistein supplementation led to an improvement in the egg production rate and feed conversion ratio, as well as an increase in egg quality. Moreover, the expression levels of senescence markers, such as β-galactosidase, P16, and P21, were decreased in the gut of genistein-treated aged laying hens. Furthermore, genistein ameliorated gut dysfunctions, such as intestinal inflammation, leaky gut, and impaired epithelial regeneration. Treg cell-derived IL-10 plays a crucial role in the genistein-induced regulation of age-related intestinal inflammation. This study demonstrates that long-term consumption of genistein improves homeostasis in the aged gut and extends the laying cycle of aged laying hens. Moreover, the link between genistein and Treg cells provides a rationale for dietary intervention against age-associated gut dysfunction.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
-
FC/FACS
-
Immunology and Microbiology
-
Veterinary Research
In Heliyon on 1 July 2022 by Kasahara, K., Sasaki, N., et al.
Compelling evidence suggests a crucial role for Foxp3+ regulatory T cells (Tregs) in the control of atherosclerosis. Although suppression of pro-inflammatory CD4+ T cell immune responses is supposed to be important for athero-protective action of Foxp3+ Tregs, few studies have provided direct evidence for this protective mechanism. We investigated the impact of Foxp3+ Treg depletion on CD4+ T cell immune responses and the development of atherosclerosis under hypercholesterolemia. We employed DEREG (depletion of regulatory T cells) mice on an atherosclerosis-prone low-density lipoprotein receptor-deficient (Ldlr -/-) background, which carry a diphtheria toxin (DT) receptor under the control of the foxp3 gene locus. In these mice, DT injection led to efficient depletion of Foxp3+ Tregs in spleen, lymph nodes and aorta. Depletion of Foxp3+ Tregs augmented CD4+ effector T cell immune responses and aggravated atherosclerosis without affecting plasma lipid profile. Notably, the proportion of pro-inflammatory IFN-γ-producing T cells were increased in spleen and aorta following Foxp3+ Treg depletion, implying that Foxp3+ Tregs efficiently regulate systemic and aortic T cell-mediated inflammatory responses under hypercholesterolemia. Unexpectedly, Foxp3+ Treg depletion resulted in an increase in anti-inflammatory IL-10-producing T cells, which was not sufficient to suppress the augmented proinflammatory T cell immune responses caused by reduced numbers of Foxp3+ Tregs. Our data indicate that Foxp3+ Tregs suppress pro-inflammatory CD4+ T cell immune responses to control atherosclerosis under hypercholesterolemia.
© 2022 The Author(s).
-
FC/FACS
-
Immunology and Microbiology
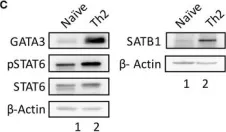
In Front Immunol on 20 April 2019 by Khare, S. P., Shetty, A., et al.
Fig.1.C

-
WB
-
Homo sapiens (Human)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 1