IL-17A-expressing lymphocytes, including Tc17 cells, are instrumental in immunity, immunopathology, and autoimmunity. We have previously shown that experimental attenuated live fungal vaccine-induced Tc17 cells are stable, long-lived without plasticity, and necessary to mediate sterilizing immunity during CD4+ T cell deficiency, which poses higher susceptibility to fungal infections. Cell metabolism is integral for T cell homeostasis but the metabolic adaptations of Tc17 cells are poorly defined. In this study, we hypothesized that effector Tc17 cells adopt high energy-yielding metabolic pathways to form stable, long-lived memory cells in vivo. Using a mouse model of attenuated fungal vaccination, we found that effector Tc17 cells were metabolically highly active with higher proliferation and protein synthesis than IFNγ+ CD8+ T (Tc1) cells. Glucose was necessary for effector Tc17 cell expansion but with less dependency during the late expansion despite the active metabolism. Contrary to established dogma, we found that the effector Tc17 cells preferentially channeled the glucose to OXPHOS than glycolysis, which was correlated with higher mitochondrial mass and membrane potential. Inhibition of OXPHOS shrunk the Tc17 responses while sparing Tc1 cell responses. Tc17 cells actively relied on OXPHOS throughout the expansion period, resisting adaptation to aerobic glycolysis. Our data showed that the effector Tc17 cells predominantly utilize glucose for metabolism through OXPHOS rather than aerobic glycolysis. Our study has implications in vaccine design to enhance the efficacy and immunotherapeutics to modulate the immunity and autoimmunity.
Copyright © 2025 John, Mudalagiriyappa, Chandrashekar and Nanjappa.
Product Citations: 28
Effector Tc17 cells resist shift from OXPHOS to aerobic glycolysis.
In Frontiers in Immunology on 2 June 2025 by John, R., Mudalagiriyappa, S., et al.
-
Immunology and Microbiology
In IScience on 15 November 2024 by Zhang, W., Cai, Z., et al.
Atherosclerosis represents a chronic inflammatory condition in arterial walls, where local immune cells significantly contribute to disease progression. This study employed various in situ immunological techniques to investigate the specific roles of aortic dendritic cell (DC) subsets in atherosclerotic animal models, distinguishing between normal and diseased immune contexts. Our findings revealed that aortic DCs, particularly the cDC1 subset, played a critical role in facilitating CD8+ T cell activation through antigen presentation. Additionally, atherosclerosis-induced increases in GM-CSF levels enhanced CCR7 expression on aortic monocyte-derived DCs, promoting their recruitment and IL-12 production for Th1 differentiation. Notably, immunizing pre-atherosclerotic mice with DC-presented antigens or transferring aortic DCs from atherosclerotic mice resulted in accelerated disease onset. This research elucidates the adaptive immune functions of aortic DCs, offering insights into the cellular mechanisms driving aortic inflammation and potential therapeutic targets for atherosclerosis management.
© 2024 The Author(s).
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Cell Death & Disease on 2 September 2024 by Ni, Q., Zhen, L., et al.
Mesenchymal stromal/stem cells (MSC) have emerged as a promising therapeutic avenue for treating autoimmune diseases, eliciting considerable interest and discussion regarding their underlying mechanisms. This study revealed the distinctive ability of human umbilical cord MSC to aggregate within the lymph nodes of mice afflicted with autoimmune diseases, but this phenomenon was not observed in healthy mice. The specific distribution is driven by the heightened expression of the CCL21-CCR7 axis in mice with autoimmune diseases, facilitating the targeted homing of MSC to the lymph nodes. Within the lymph nodes, MSC exhibit a remarkable capacity to modulate Th17 cell function, exerting a pronounced anti-inflammatory effect. Transplanted MSC stimulates the secretion of L-amino-acid oxidase (LAAO), a response triggered by elevated levels of tumor necrosis factor-α (TNF-α) in mice with autoimmune diseases through the NF-κB pathway. The presence of LAAO is indispensable for the efficacy of MSC, as it significantly contributes to the inhibition of Th17 cells. Furthermore, LAAO-derived indole-3-pyruvic acid (I3P) serves as a potent suppressor of Th17 cells by activating the aryl hydrocarbon receptor (AHR) pathway. These findings advance our understanding of the global immunomodulatory effects exerted by MSC, providing valuable information for optimizing therapeutic outcomes.
© 2024. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Cell Biology
Sphingolipid biosynthesis is essential for metabolic rewiring during TH17 cell differentiation.
In Science Advances on 26 April 2024 by Abimannan, T., Parthibane, V., et al.
T helper 17 (TH17) cells are implicated in autoimmune diseases, and several metabolic processes are shown to be important for their development and function. In this study, we report an essential role for sphingolipids synthesized through the de novo pathway in TH17 cell development. Deficiency of SPTLC1, a major subunit of serine palmitoyl transferase enzyme complex that catalyzes the first and rate-limiting step of de novo sphingolipid synthesis, impaired glycolysis in differentiating TH17 cells by increasing intracellular reactive oxygen species (ROS) through enhancement of nicotinamide adenine dinucleotide phosphate oxidase 2 activity. Increased ROS leads to impaired activation of mammalian target of rapamycin C1 and reduced expression of hypoxia-inducible factor 1-alpha and c-Myc-induced glycolytic genes. SPTLCI deficiency protected mice from developing experimental autoimmune encephalomyelitis and experimental T cell transfer colitis. Our results thus show a critical role for de novo sphingolipid biosynthetic pathway in shaping adaptive immune responses with implications in autoimmune diseases.
-
Mus musculus (House mouse)
-
Biochemistry and Molecular biology
-
Cell Biology
IL-10 constrains sphingolipid metabolism to limit inflammation.
In Nature on 1 March 2024 by York, A. G., Skadow, M. H., et al.
Interleukin-10 (IL-10) is a key anti-inflammatory cytokine that can limit immune cell activation and cytokine production in innate immune cell types1. Loss of IL-10 signalling results in life-threatening inflammatory bowel disease in humans and mice-however, the exact mechanism by which IL-10 signalling subdues inflammation remains unclear2-5. Here we find that increased saturated very long chain (VLC) ceramides are critical for the heightened inflammatory gene expression that is a hallmark of IL-10 deficiency. Accordingly, genetic deletion of ceramide synthase 2 (encoded by Cers2), the enzyme responsible for VLC ceramide production, limited the exacerbated inflammatory gene expression programme associated with IL-10 deficiency both in vitro and in vivo. The accumulation of saturated VLC ceramides was regulated by a decrease in metabolic flux through the de novo mono-unsaturated fatty acid synthesis pathway. Restoring mono-unsaturated fatty acid availability to cells deficient in IL-10 signalling limited saturated VLC ceramide production and the associated inflammation. Mechanistically, we find that persistent inflammation mediated by VLC ceramides is largely dependent on sustained activity of REL, an immuno-modulatory transcription factor. Together, these data indicate that an IL-10-driven fatty acid desaturation programme rewires VLC ceramide accumulation and aberrant activation of REL. These studies support the idea that fatty acid homeostasis in innate immune cells serves as a key regulatory node to control pathologic inflammation and suggests that 'metabolic correction' of VLC homeostasis could be an important strategy to normalize dysregulated inflammation caused by the absence of IL-10.
© 2024. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Biochemistry and Molecular biology
-
Cell Biology
-
Immunology and Microbiology
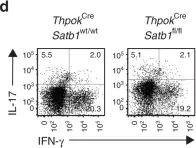
In Nat Commun on 1 February 2019 by Yasuda, K., Kitagawa, Y., et al.
Fig.5.D

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 3
In Nat Commun on 1 February 2019 by Yasuda, K., Kitagawa, Y., et al.
Fig.1.C

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 3
In Nat Commun on 1 February 2019 by Yasuda, K., Kitagawa, Y., et al.
Fig.1.D

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 3