The aim of the present study was to verify whether overexpression of CXC receptor 4 (CXCR4) promotes the invasion and migration of non-small cell lung cancer (NSCLC) via epidermal growth factor receptor (EGFR) and matrix metallopeptidase-9 (MMP-9), and to detect the association between CXCR4, EGFR and MMP-9. The effects of overexpression of CXCR4 on lung cancer cell functions were investigated by migration and invasion assays. Western blotting and zymograph assays were used to analyze the protein expression levels of EGFR and the production of MMP-9, respectively. Immunohistochemistry was applied to analyze the expression of EGFR, CXCR4 and MMP-9 in NSCLC. Statistical analyses were used to detect the associations among EGFR, CXCR4 and MMP-9 in NSCLC. Finally, survival analyses were performed. CXCR4 overexpression enhanced cell motility and invasion. CXCR4 also promoted expression of EGFR and elevated MMP-9 production. CXCR4, EGFR and MMP-9 were highly expressed in NSCLC, and were not identified as associated with age and sex (P>0.05). However, they were associated with tumor differentiation and lymph node metastasis (P<0.05). CXCR4, EGFR and CXCR4 expression were positively associated with one another in NSCLC (P<0.05). In addition, patients with positive expression of CXCR4, EGFR or MMP-9 in tumors exhibited significantly shorter overall survival compared with those with negative expression (P<0.05). In conclusion, CXCR4 overexpression enhanced cell motility and invasion via EGFR and MMP-9. CXCR4, EGFR and MMP-9 were identified as highly expressed in NSCLC, and there was positive correlation among them.
Product Citations: 2
In Oncology Letters on 1 December 2017 by Zuo, J., Wen, M., et al.
-
Homo sapiens (Human)
-
Cancer Research
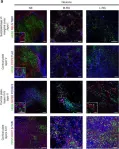
In Nature Communications on 23 March 2015 by Edri, R., Yaffe, Y., et al.
Decoding heterogeneity of pluripotent stem cell (PSC)-derived neural progeny is fundamental for revealing the origin of diverse progenitors, for defining their lineages, and for identifying fate determinants driving transition through distinct potencies. Here we have prospectively isolated consecutively appearing PSC-derived primary progenitors based on their Notch activation state. We first isolate early neuroepithelial cells and show their broad Notch-dependent developmental and proliferative potential. Neuroepithelial cells further yield successive Notch-dependent functional primary progenitors, from early and midneurogenic radial glia and their derived basal progenitors, to gliogenic radial glia and adult-like neural progenitors, together recapitulating hallmarks of neural stem cell (NSC) ontogeny. Gene expression profiling reveals dynamic stage-specific transcriptional patterns that may link development of distinct progenitor identities through Notch activation. Our observations provide a platform for characterization and manipulation of distinct progenitor cell types amenable for developing streamlined neural lineage specification paradigms for modelling development in health and disease.
-
IF
-
ICC-IF
-
Homo sapiens (Human)
-
Stem Cells and Developmental Biology
In Nat Commun on 23 March 2015 by Edri, R., Yaffe, Y., et al.
Fig.3.A

-
ICC-IF
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 1