Oncolytic viruses are a promising approach for cancer treatment where viruses selectively target and kill cancer cells while also stimulating an immune response. Among viruses with this ability, bovine herpesvirus-1 (BoHV-1) has several advantages, including observations suggesting it may not require viral replication for its anti-cancer effects. We previously demonstrated that binding and penetration of enveloped virus particles are sufficient to trigger intrinsic and innate immune signaling in normal cells, while other groups have published the efficacy of non-replicating viruses as viable immunotherapies in different cancer models. In this work, we definitively show that live and UV-inactivated (UV) (non-replicating) BoHV-1-based regimens extend survival of tumor-bearing mice to similar degrees and induce infiltration of similar immune cell populations, with the exception of neutrophils. Transcriptomic analysis of tumors treated with either live or UV BoHV-1-based regimens revealed similar pathway enrichment and a subset of overlapping differentially regulated genes, suggesting live and UV BoHV-1 have similar mechanisms of activity. Last, we present a gene signature across our in vitro and in vivo models that could potentially be used to validate new BoHV-1 therapeutics. This work contributes to the growing body of literature showing that replication may not be necessary for therapeutic efficacy of viral immunotherapies.
© 2024 The Author(s).
Product Citations: 25
Virus replication is not required for oncolytic bovine herpesvirus-1 immunotherapy.
In Molecular Therapy. Oncology on 19 December 2024 by Baracuhy, E. M., Cormier, O., et al.
-
Immunology and Microbiology
-
Veterinary Research
In IScience on 15 November 2024 by Zhang, W., Cai, Z., et al.
Atherosclerosis represents a chronic inflammatory condition in arterial walls, where local immune cells significantly contribute to disease progression. This study employed various in situ immunological techniques to investigate the specific roles of aortic dendritic cell (DC) subsets in atherosclerotic animal models, distinguishing between normal and diseased immune contexts. Our findings revealed that aortic DCs, particularly the cDC1 subset, played a critical role in facilitating CD8+ T cell activation through antigen presentation. Additionally, atherosclerosis-induced increases in GM-CSF levels enhanced CCR7 expression on aortic monocyte-derived DCs, promoting their recruitment and IL-12 production for Th1 differentiation. Notably, immunizing pre-atherosclerotic mice with DC-presented antigens or transferring aortic DCs from atherosclerotic mice resulted in accelerated disease onset. This research elucidates the adaptive immune functions of aortic DCs, offering insights into the cellular mechanisms driving aortic inflammation and potential therapeutic targets for atherosclerosis management.
© 2024 The Author(s).
-
Mus musculus (House mouse)
-
Immunology and Microbiology
Integration Analysis of Single-Cell Multi-Omics Reveals Prostate Cancer Heterogeneity.
In Advanced Science (Weinheim, Baden-Wurttemberg, Germany) on 1 May 2024 by Bian, X., Wang, W., et al.
Prostate cancer (PCa) is an extensive heterogeneous disease with a complex cellular ecosystem in the tumor microenvironment (TME). However, the manner in which heterogeneity is shaped by tumors and stromal cells, or vice versa, remains poorly understood. In this study, single-cell RNA sequencing, spatial transcriptomics, and bulk ATAC-sequence are integrated from a series of patients with PCa and healthy controls. A stemness subset of club cells marked with SOX9highARlow expression is identified, which is markedly enriched after neoadjuvant androgen-deprivation therapy (ADT). Furthermore, a subset of CD8+CXCR6+ T cells that function as effector T cells is markedly reduced in patients with malignant PCa. For spatial transcriptome analysis, machine learning and computational intelligence are comprehensively utilized to identify the cellular diversity of prostate cancer cells and cell-cell communication in situ. Macrophage and neutrophil state transitions along the trajectory of cancer progression are also examined. Finally, the immunosuppressive microenvironment in advanced PCa is found to be associated with the infiltration of regulatory T cells (Tregs), potentially induced by an FAP+ fibroblast subset. In summary, the cellular heterogeneity is delineated in the stage-specific PCa microenvironment at single-cell resolution, uncovering their reciprocal crosstalk with disease progression, which can be helpful in promoting PCa diagnosis and therapy.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
-
Mus musculus (House mouse)
-
Cancer Research
In Cell Reports on 23 April 2024 by Houbaert, D., Nikolakopoulos, A. P., et al.
Lymphatic endothelial cells (LECs) of the lymph node (LN) parenchyma orchestrate leukocyte trafficking and peripheral T cell dynamics. T cell responses to immunotherapy largely rely on peripheral T cell recruitment in tumors. Yet, a systematic and molecular understanding of how LECs within the LNs control T cell dynamics under steady-state and tumor-bearing conditions is lacking. Intravital imaging combined with immune phenotyping shows that LEC-specific deletion of the essential autophagy gene Atg5 alters intranodal positioning of lymphocytes and accrues their persistence in the LNs by increasing the availability of the main egress signal sphingosine-1-phosphate. Single-cell RNA sequencing of tumor-draining LNs shows that loss of ATG5 remodels niche-specific LEC phenotypes involved in molecular pathways regulating lymphocyte trafficking and LEC-T cell interactions. Functionally, loss of LEC autophagy prevents recruitment of tumor-infiltrating T and natural killer cells and abrogates response to immunotherapy. Thus, an LEC-autophagy program boosts immune-checkpoint responses by guiding systemic T cell dynamics.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
-
Mus musculus (House mouse)
-
Cell Biology
-
Immunology and Microbiology
In Cell Reports Medicine on 16 January 2024 by Wallace, R. P., Refvik, K. C., et al.
Immunogenic biologics trigger an anti-drug antibody (ADA) response in patients that reduces efficacy and increases adverse reactions. Our laboratory has shown that targeting protein antigen to the liver microenvironment can reduce antigen-specific T cell responses; herein, we present a strategy to increase delivery of otherwise immunogenic biologics to the liver via conjugation to a synthetic mannose polymer, p(Man). This delivery leads to reduced antigen-specific T follicular helper cell and B cell responses resulting in diminished ADA production, which is maintained throughout subsequent administrations of the native biologic. We find that p(Man)-antigen treatment impairs the ADA response against recombinant uricase, a highly immunogenic biologic, without a dependence on hapten immunodominance or control by T regulatory cells. We identify increased T cell receptor signaling and increased apoptosis and exhaustion in T cells as effects of p(Man)-antigen treatment via transcriptomic analyses. This modular platform may enhance tolerance to biologics, enabling long-term solutions for an ever-increasing healthcare problem.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
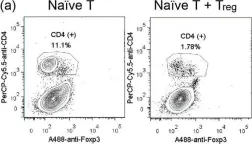
In Sci Rep on 20 October 2016 by Chen, Y. L., Chen, Y. T., et al.
Fig.3.A

-
FC/FACS
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 2
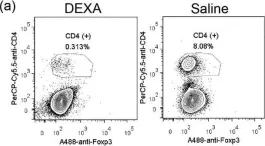
In Sci Rep on 20 October 2016 by Chen, Y. L., Chen, Y. T., et al.
Fig.6.A

-
FC/FACS
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 2