Chromosome instability, a hallmark of lung cancer, is a driving mechanism for hexavalent chromium [Cr(VI)] carcinogenesis in humans. Cr(VI) induces structural and numerical chromosome instability in human lung cells by inducing DNA double-strand breaks and inhibiting homologous recombination repair and causing spindle assembly checkpoint (SAC) bypass and centrosome amplification. Great whales are long-lived species with long-term exposures to Cr(VI) and accumulate Cr in their tissue, but exhibit a low incidence of cancer. Data show Cr(VI) induces fewer chromosome aberrations in whale cells after acute Cr(VI) exposure suggesting whale cells can evade Cr(VI)-induced chromosome instability. However, it is unknown if whales can evade Cr(VI)-induced chromosome instability. Thus, we tested the hypothesis that whale cells resist Cr(VI)-induced loss of homologous recombination repair activity and increased SAC bypass and centrosome amplification. We found Cr(VI) induces similar amounts of DNA double-strand breaks after acute (24 h) and prolonged (120 h) exposures in whale lung cells, but does not inhibit homologous recombination repair, SAC bypass, or centrosome amplification, and does not induce chromosome instability. These data indicate whale lung cells resist Cr(VI)-induced chromosome instability, the major driver for Cr(VI) carcinogenesis at a cellular level, consistent with observations that whales are resistant to cancer.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Product Citations: 44
In Toxicological Sciences on 29 April 2024 by Lu, H., Toyoda, J. H., et al.
-
Genetics
In Nucleic Acids Research on 11 December 2023 by Xu, Z., Flensburg, C., et al.
Molnupiravir (EIDD-2801) is an antiviral that received approval for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) infection. Treatment of bacteria or cell lines with the active form of molnupiravir, β-d-N4-hydroxycytidine (NHC, or EIDD-1931), induces mutations in DNA. Yet these results contrast in vivo genotoxicity studies conducted during registration of the drug. Using a CRISPR screen, we found that inactivating the pyrimidine salvage pathway component uridine-cytidine kinase 2 (Uck2) renders cells more tolerant of NHC. Short-term exposure to NHC increased the mutation rate in a mouse myeloid cell line, with most mutations being T:A to C:G transitions. Inactivating Uck2 impaired the mutagenic activity of NHC, whereas over-expression of Uck2 enhanced mutagenesis. UCK2 is upregulated in many cancers and cell lines. Our results suggest differences in ribonucleoside metabolism contribute to the variable mutagenicity of NHC observed in cancer cell lines and primary tissues.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
-
Mus musculus (House mouse)
-
Biochemistry and Molecular biology
-
Genetics
In Frontiers in Immunology on 20 October 2023 by Lavandoski, P., Pierdoná, V., et al.
Eotaxin-1/CCL11 is a pivotal chemokine crucial for eosinophil homing to the lungs of asthmatic patients. Recent studies also suggest that CCL11 is involved in the aging process, as it is upregulated in elderly, and correlated with shorter telomere length in leukocytes from asthmatic children. Despite its potential pro-aging effects, the precise contribution of CCL11 and the underlying mechanisms involved in the promotion of cellular senescence remains unclear. Therefore, the primary goal of this study was to explore the role of CCL11 on senescence development and the signaling pathways activated by this chemokine in lung fibroblasts.
To investigate the targets potentially modulated by CCL11, we performed an in silico analysis using PseudoCell. We validated in vitro the activation of these targets in the human lung fibroblast cell line MRC-5 following rhCCL11 exposure. Finally, we performed differential gene expression analysis in human airway epithelial cells of asthmatic patients to assess CCL11 signaling and activation of additional senescent markers.
Our study revealed that eotaxin-1/CCL11 promote reactive oxygen secretion (ROS) production in lung fibroblasts, accompanied by increased activation of the DNA damage response (DDR) and p-TP53 and γH2AX. These modifications were accompanied by cellular senescence promotion and increased secretion of senescence-associated secretory phenotype inflammatory cytokines IL-6 and IL-8. Furthermore, our data show that airway epithelial lung cells from atopic asthmatic patients overexpress CCL11 along with aging markers such as CDKN2A (p16INK4a) and SERPINE1.
These findings provide new insights into the mechanisms underlying the pro-aging effects of CCL11 in the lungs of asthmatic patients. Understanding the role of CCL11 on senescence development may have important implications for the treatment of age-related lung diseases, such as asthma.
Copyright © 2023 Lavandoski, Pierdoná, Maurmann, Grun, Guma and Barbé-Tuana.
-
Homo sapiens (Human)
-
Immunology and Microbiology
Replication stress in activated human NK cells induces sensitivity to apoptosis
Preprint on BioRxiv : the Preprint Server for Biology on 7 September 2023 by Guilz, N. C., Ahn, Y., et al.
Natural killer cells are innate immune effectors that kill virally infected or malignant cells. Natural killer cell deficiency (NKD) occurs when NK cell development or function are impaired, and individuals with NKD are susceptible to severe and recurrent viral infections. Several gene deficiencies result in NKD, including variants in MCM4, GINS1, MCM10 and GINS4, which are components of the CDC45-MCM-GINS (CMG) helicase. The CMG helicase unwinds DNA during replication and is expressed in any actively proliferating cell. NK cells are more strongly impacted by mutational deficiencies in helicase proteins than other lymphocytes, though the mechanisms underlying this susceptibility are not completely understood. NK cells from individuals with NKD as a result of helicase deficiency have increased DNA damage, cell cycle arrest, and replication stress. Here, we induced replication stress in activated mature NK cells or T cells by chemical methods, using aphidicolin, and through shRNA knockdown of MCM10 in an NK cell line. We found that the CD56 bright subset of NK cells accumulates more DNA damage and replication stress during activation than CD56 dim NK cells or activated T cells. Aphidicolin treatment increases apoptosis of CD56 bright NK cells through increased pan-caspase expression and decreases perforin expression in surviving cells. This effect is modeled by shRNA mediated knockdown of MCM10, thus linking decreased helicase protein expression to replication stress and impaired NK cell function. These findings show that sensitivity to replication stress affects human NK cell survival and function and can contribute to NK cell deficiency.
-
Homo sapiens (Human)
In Radiation and Environmental Biophysics on 1 August 2023 by López-Riego, M., Płódowska, M., et al.
Candidate ionising radiation exposure biomarkers must be validated in humans exposed in vivo. Blood from patients undergoing positron emission tomography-computed tomography scan (PET-CT) and skeletal scintigraphy (scintigraphy) was drawn before (0 h) and after (2 h) the procedure for correlation analyses of the response of selected biomarkers with radiation dose and other available patient information. FDXR, CDKN1A, BBC3, GADD45A, XPC, and MDM2 expression was determined by qRT-PCR, DNA damage (γH2AX) by flow cytometry, and reactive oxygen species (ROS) levels by flow cytometry using the 2', 7'-dichlorofluorescein diacetate test in peripheral blood mononuclear cells (PBMC). For ROS experiments, 0- and 2-h samples were additionally exposed to UVA to determine whether diagnostic irradiation conditioned the response to further oxidative insult. With some exceptions, radiological imaging induced weak γH2AX foci, ROS and gene expression fold changes, the latter with good coherence across genes within a patient. Diagnostic imaging did not influence oxidative stress in PBMC successively exposed to UVA. Correlation analyses with patient characteristics led to low correlation coefficient values. γH2AX fold change, which correlated positively with gene expression, presented a weak positive correlation with injected activity, indicating a radiation-induced subtle increase in DNA damage and subsequent activation of the DNA damage response pathway. The exposure discrimination potential of these biomarkers in the absence of control samples as frequently demanded in radiological emergencies, was assessed using raw data. These results suggest that the variability of the response in heterogeneous populations might complicate identifying individuals exposed to low radiation doses.
© 2023. The Author(s).
-
Homo sapiens (Human)
-
Genetics
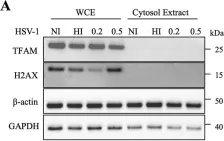
In MBio on 21 December 2021 by Berry, N., Suspène, R., et al.
Fig.4.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from MBio by CiteAb, provided under a CC-BY license
Image 1 of 1