Recombinant granulocyte colony-stimulating factor (G-CSF) is the most commonly used agent for treating neutropenia and mobilizing hematopoietic stem cells (HSCs) for transplantation. However, some patients do not respond effectively to the currently used mobilization protocols. To address this, new therapeutic approaches are needed. A potential strategy is pharmacological induction of endogenous mobilizing factors via cobalt protoporphyrin IX (CoPP). CoPP mobilizes HSCs and granulocytes by increasing endogenous G-CSF, though optimal dosing and potential side effects remain unclear. Our study aimed to optimize CoPP dosing and timing, and assess its safety in mobilizing cells from bone marrow to blood.
Mice were treated with different doses of CoPP, and blood cell counts, cytokine concentrations, and organ damage markers were evaluated at various time points after injection.
Our results show that CoPP exerts a dose-dependent mobilizing effect, with the highest G-CSF levels and number of mobilized leukocytes observed in mice treated with 10 mg/kg of CoPP. While there were no severe adverse effects, there were mild fluctuations in markers of organ function, including a reduction in blood urea nitrogen (BUN) and glucose levels during the five days of administration. Additionally, although most parameters normalized within 30 days, the decrease in BUN persisted. Mice experienced short-term weight loss following CoPP administration, but they regained their initial weight within two weeks.
This study demonstrates that CoPP mobilizes cells from the bone marrow to the blood in a dose-dependent manner, with mild side effects, including temporary changes in biochemical markers and a sustained reduction in BUN levels.
© 2025. The Author(s).
Product Citations: 66
In Pharmacological Reports : PR on 9 July 2025 by Bednarz, A., Kozuch, P., et al.
-
Biochemistry and Molecular biology
-
Cell Biology
In Investigative Ophthalmology & Visual Science on 1 July 2025 by Cui, X., Yi, C., et al.
The purpose of this study was to understand how the gut microbial system responds to retinal injury.
Adult C57BL/6J mice were subjected to retinal laser burns or hypotony-induced retinal detachment (RD). One, 4, and 24 hours later, gut permeability (8 male mice and 8 female mice) was assessed using Evan's blue assay and the expression of ZO-1 in intestinal epithelial cells was examined by immunofluorescence. Circulating immune cells were evaluated by flow cytometry. The feces from control and lasered mice (n = 8) were collected under strict sterile conditions and processed for 16S DNA paired-end sequencing using the Illumina platform. The impact of gut dysbiosis on retinal wound healing was evaluated following treatment with Peros antibiotics (n = 8). Retinal pathologies were examined by immunohistochemistry.
Retinal laser injury significantly altered gut microbial profiles within 1 hour (β-diversity, multi-response permutation procedure [MRPP], P = 0.05). The abundance of Lignipirellula and Faecalibacterium was 100- and 6.67-fold lower, and the abundance of Akkermansia and Colidextribacter was 3.65- and 17.72-fold higher than non-lasered controls, respectively. Retinal laser burns and RD, not sham surgery, increased gut permeability at 1 hour and 4 hours by 3.82- and 24.76-fold, respectively, disrupted intestinal epithelial ZO-1 expression, accompanied by an increased population of circulating neutrophils and monocytes (P < 0.01) at 1 hour and 4 hours. Antibiotic treatment attenuated laser-/RD-induced gut permeability and the increased neutrophils and monocytes (in RD, P < 0.05). Antibiotic treatment also significantly reduced the severity of laser-induced choroidal neovascularization (CNV; P < 0.001) and RD-mediated photoreceptor apoptosis (P < 0.01), and suppressed Gr-1+ neutrophils (CNV, P < 0.001) and Iba-1+ cell infiltration (P < 0.001).
A retina-gut axis exists. Retinal injury induces rapid gut microbial alteration, which in turn modulates innate immune cell activation and regulates the wound healing response.
-
Immunology and Microbiology
-
Neuroscience
In International Journal of Biological Sciences on 19 May 2025 by Huo, S., Wang, M., et al.
The macrophage-cardiomyocyte crosstalk as a potential intervention target for diabetic cardiomyopathy (DCM) remains deeper exploration. We found S100A9, as an immunoinflammatory mediator, was up-regulated in cardiomyocytes and macrophages in diabetic heart by single-cell analysis. Furthermore, F4/80+CCR2+S100A9+ macrophages in peripheral blood and heart both increased in diabetic mice. S100A9 blocking by paquinimod or macrophage depletion (clodronate) alleviated diabetes-induced cardiac dysfunction, inflammatory macrophage infiltration, serum pro-inflammatory cytokines. More importantly, diabetic cardiac dysfunction, myocardial remodeling, and inflammation could be suppressed by macrophage specific S100A9 knockout (S100a9flox/floxLyz2-Cre). S100A9 activation led to excessive mitochondrial fission, decreased mitophagy flux, and elevated mitochondrial oxidative stress. In addition, proteomics and transcription factor profiling array unveiled S100A9 activated STAT3 in cardiomyocytes. Nevertheless, these effects were mitigated by STAT3(Y705F) mutation, STAT3 knockdown, or paquinimod. Our study highlights macrophage-derived S100A9 as a critical mediator for impaired mitochondrial quality control in diabetic cardiac dysfunction, and targeting S100A9 represents a promising therapeutic target.
© The author(s).
-
Cardiovascular biology
-
Cell Biology
-
Immunology and Microbiology
USP2 inhibition unleashes CD47-restrained phagocytosis and enhances anti-tumor immunity.
In Nature Communications on 16 May 2025 by Dai, P., Sun, Y., et al.
The CD47/SIRPα axis conveys a 'don't eat me' signal, thereby thwarting the phagocytic clearance of tumor cells. Although blocking antibodies targeting CD47 have demonstrated promising anti-tumor effects in preclinical models, clinical trials involving human cancer patients have not yielded ideal results. Exploring the regulatory mechanisms of CD47 is imperative for devising more efficacious combinational therapies. Here, we report that inhibiting USP2 prompts CD47 degradation and reshapes the tumor microenvironment (TME), thereby enhancing anti-PD-1 immunotherapy. Mechanistically, USP2 interacts with CD47, stabilizing it through deubiquitination. USP2 inhibition destabilizes CD47, thereby boosting macrophage phagocytosis. Single-cell RNA sequencing shows USP2 inhibition reprograms TME, evidenced by increasing M1 macrophages and CD8+ T cells while reducing M2 macrophages. Combining ML364 with anti-PD-1 reduces tumor burden in mouse models. Clinically, low USP2 expression predicts a better response to anti-PD-1 treatment. Our findings uncover the regulatory mechanism of CD47 by USP2 and targeting this axis boosts anti-tumor immunity.
© 2025. The Author(s).
-
Cancer Research
-
Immunology and Microbiology
In Immunology on 1 February 2025 by Xue, M., Yang, R., et al.
Psoriasis is a chronic inflammatory skin disease characterised by inflammatory cell infiltration, keratinocyte hyperproliferation and increased neovascularization. Despite extensive research, the precise mechanisms underlying psoriasis pathology and treatment strategies remain unclear because of a complex aetiology and disease progression. Hence, in this study, we aimed to identify potential therapeutic targets for psoriasis and explore their effects on disease progression. We observed that G protein-coupled receptor LGR4 attenuates psoriasis progression. Bioinformatics analysis of publicly available clinical data revealed lower LGR4 expression in the skin lesions of patients with psoriasis than in their non-lesioned skin. Both in vitro (HaCaT cell) and in vivo (mouse) models confirmed this phenomenon. The Lgr4-knockout mouse model further confirmed that LGR4 plays a positive role in psoriasis progression. Specifically, Lgr4 knockout promoted the secretion of inflammatory factors, accumulation of local immunocyte infiltration in skin lesions, and keratinocyte proliferation. In conclusion, we demonstrated that LGR4 is critical to limiting psoriasis progression, suggesting that it is a viable target for the clinical management of this skin condition.
© 2024 John Wiley & Sons Ltd.
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In J Neuroinflammation on 13 May 2023 by Kak, G., Van Roy, Z., et al.
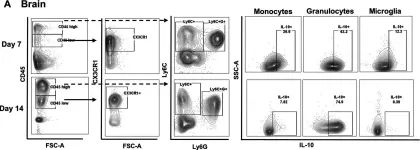
Fig.1.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from J Neuroinflammation by CiteAb, provided under a CC-BY license
Image 1 of 3
In JCI Insight on 8 February 2021 by She, L., Barrera, G. D., et al.
Fig.7.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from JCI Insight by CiteAb, provided under a CC-BY license
Image 1 of 3
In Oncotarget on 20 October 2015 by Patel, M. R., Jacobson, B. A., et al.
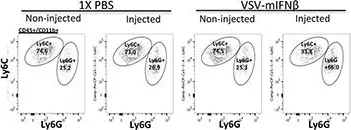
Fig.6.G

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 3