Inflammatory environments induce the generation of dysfunctional IFNγ+T-bet+FOXP3+ Th1-like Tregs, which show defective function and are found in autoimmune conditions including multiple sclerosis (MS). The pathways that control the generation of Th1-like Tregs are not well understood. Sphingosine-1-phosphate (S1P) signalling molecules are upregulated in Th1-like Tregs, and in vivo S1P inhibition with Fingolimod (FTY720) inhibits the expression of genes responsible for Treg plasticity in MS patients. However, the underlying mechanisms are unknown. Here we show that S1P signalling inhibition by FTY720 inhibits the generation of Th1-like Tregs and rescues their suppressive function. These effects are mediated by a decrease in mTORC1 signalling and reversal of the mitochondrial uncoupling that Tregs undergo during their reprogramming into Th1-like Tregs in vitro. Finally, these results are validated in in vivo-generated Th1-like Tregs, as Tregs from MS patients treated with FTY720 display decreased Th1-like Treg frequency, increased suppressive function and mitochondrial metabolism rebalance. These results highlight the involvement of mitochondrial uncoupling in Treg reprogramming and identify S1P signalling inhibition as a target to suppress the generation of dysfunctional Th1-like Tregs.
© 2024 The Author(s). Immunology published by John Wiley & Sons Ltd.
Product Citations: 40
In Immunology on 1 January 2025 by Coulombeau, R., Selck, C., et al.
-
Immunology and Microbiology
-
Cell Biology
In Haematologica on 1 April 2024 by Favaloro, J., Bryant, C. E., et al.
Multiple myeloma (MM) is an incurable disease of the bone marrow (BM) characterized by the uncontrolled proliferation of neoplastic plasma cells. While CD8+ T cells have an established role in disease control, few studies have focused on these cells within the MM tumor microenvironment (TME). We analyzed CD8+ T cells in the BM and peripheral blood (PB) of untreated patients with MM and non-myeloma controls using flow cytometry, mass cytometry and single-cell RNA sequencing, using several novel bioinformatics workflows. Inter-tissue differences were most evident in the differential expression of Granzymes B and K, which were strongly associated with two distinct subsets of CD8+ T cells delineated by the expression of CD69, accounting for roughly 50% of BM-CD8+ T cells of all assessed cohorts. While few differences were observable between health and disease in the BM-restricted CD8CD69+ T-cell subset, the CD8+CD69- T-cell subset in the BM of untreated MM patients demonstrated increased representation of highly differentiated effector cells and evident compositional parallels between the PB, absent in age-matched controls, where a marked reduction of effector cells was observed. We demonstrate the transcriptional signature of BM-CD8+ T cells from patients with MM more closely resembles TCR-activated CD8+ T cells from age-matched controls than their resting counterparts.
-
Homo sapiens (Human)
-
Cancer Research
-
Cardiovascular biology
-
Immunology and Microbiology
In Clinical Cancer Research on 1 September 2023 by Roschewski, M., Patel, M. R., et al.
Novel targeted and immunotherapies have improved outcomes in relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL), but toxicities limit widespread use. The selective Bruton tyrosine kinase (BTK) inhibitor acalabrutinib has activity in patients with R/R DLBCL but durable responses are uncommon. STAT3 inhibition has demonstrated clinical activity in DLBCL.
Final results of the phase I study of acalabrutinib plus STAT3 inhibitor (danvatirsen; AZD9150) in patients with R/R DLBCL are reported. Danvatirsen 200 mg intravenous infusion [Days 1, 3, 5 (Cycle 1); weekly infusions starting Day 8, Cycle 1] was administered in combination with oral acalabrutinib 100 mg twice daily until progressive disease (PD) or unacceptable toxicity. Primary endpoints were safety and tolerability. Secondary endpoints included efficacy, pharmacokinetics, and immunogenicity.
Seventeen patients received combination treatment. One dose-limiting toxicity (Grade 3 liver transaminase) occurred in 1 patient. The most common reason for treatment discontinuation was PD (65%). In evaluable patients (n = 17), objective response rate was 24%; median duration of response was 1.9 months. All responders with available DLBCL cell-of-origin data were either activated B-cell or nongerminal center B-cell like subtype. Genetic subtype did not correlate with response. Baseline and longitudinal plasma cell-free DNA (cfDNA) concentrations were mostly higher in nonresponding patients. cfDNA changes were generally concordant with imaging. Pretreatment circulating B-cell levels were higher in responders versus nonresponders.
Targeting both STAT3 and BTK in combination is safe and tolerable but efficacy is limited in R/R DLBCL. Results support evaluation of circulating tumor DNA as a biomarker for clinical response.
©2023 The Authors; Published by the American Association for Cancer Research.
-
Homo sapiens (Human)
-
Cancer Research
-
Genetics
-
Immunology and Microbiology
In Frontiers in Immunology on 25 October 2022 by Paris, O., Mennechet, F. J. D., et al.
Innate lymphoid cells (ILCs), the complements of diverse CD4 T helper cells, help maintain tissue homeostasis by providing a link between innate and adaptive immune responses. While pioneering studies over the last decade have advanced our understanding how ILCs influence adaptive immune responses to pathogens, far less is known about whether the adaptive immune response feeds back into an ILC response. In this study, we isolated ILCs from blood of healthy donors, fine-tuned culture conditions, and then directly challenged them with human adenoviruses (HAdVs), with HAdVs and host defense proteins (HDPs) or neutralizing antibodies (NAbs), to mimic interactions in a host with pre-existing immunity. Additionally, we developed an ex vivo approach to identify how bystander ILCs respond to the uptake of HAdVs ± neutralizing antibodies by monocyte-derived dendritic cells. We show that ILCs take up HAdVs, which induces phenotypic maturation and cytokine secretion. Moreover, NAbs and HDPs complexes modified the cytokine profile generated by ILCs, consistent with a feedback loop for host antiviral responses and potential to impact adenovirus-based vaccine efficacy.
Copyright © 2022 Paris, Mennechet and Kremer.
-
FC/FACS
-
Immunology and Microbiology
In The Journal of Clinical Investigation on 17 October 2022 by Asashima, H., Axisa, P. P., et al.
B cell depletion in patients with relapsing-remitting multiple sclerosis (RRMS) markedly prevents new MRI-detected lesions and disease activity, suggesting the hypothesis that altered B cell function leads to the activation of T cells driving disease pathogenesis. Here, we performed comprehensive analyses of CD40 ligand- (CD40L-) and IL-21-stimulated memory B cells from patients with MS and healthy age-matched controls, modeling the help of follicular helper T cells (Tfh cells), and found a differential gene expression signature in multiple B cell pathways. Most striking was the impaired TIGIT expression on MS-derived B cells mediated by dysregulation of the transcription factor TCF4. Activated circulating Tfh cells (cTfh cells) expressed CD155, the ligand of TIGIT, and TIGIT on B cells revealed their capacity to suppress the proliferation of IL-17-producing cTfh cells via the TIGIT/CD155 axis. Finally, CCR6+ cTfh cells were significantly increased in patients with MS, and their frequency was inversely correlated with that of TIGIT+ B cells. Together, these data suggest that the dysregulation of negative feedback loops between TIGIT+ memory B cells and cTfh cells in MS drives the activated immune system in this disease.
-
FC/FACS
-
Immunology and Microbiology
In Nature on 1 April 2021 by Platten, M., Bunse, L., et al.
Fig.4.C

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Nature by CiteAb, provided under a CC-BY license
Image 1 of 2
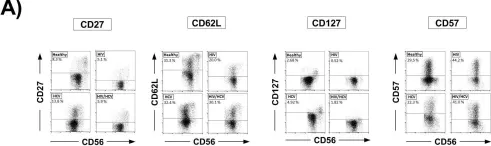
In PLoS One on 6 April 2017 by Kaczmarek, D. J., Kokordelis, P., et al.
Fig.3.A

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 2